Overview
An implantable cardioverter-defibrillator (ICD) — a pager-sized device — is placed in your chest to reduce your risk of dying if the lower chambers of your heart (ventricles) go into a dangerous rhythm and stop beating effectively (cardiac arrest).
You might need an ICD if you have a dangerously fast heartbeat (ventricular tachycardia) or a chaotic heartbeat that keeps your heart from supplying enough blood to the rest of your body (ventricular fibrillation).
ICDs detect and stop abnormal heartbeats (arrhythmias). The device continuously monitors your heartbeat and delivers electrical pulses to restore a normal heart rhythm when necessary.
An ICD differs from a pacemaker — another implantable device used to help control abnormal heart rhythms.
Why it's done
You've likely seen TV shows in which hospital workers "shock" an unconscious person out of cardiac arrest with electrified paddles. An ICD does the same thing only internally and automatically when it detects an abnormal heart rhythm.
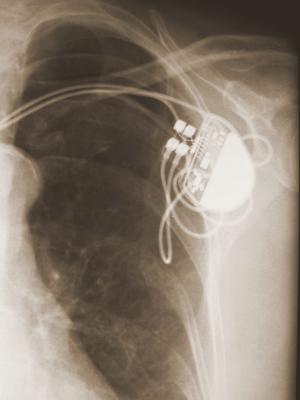
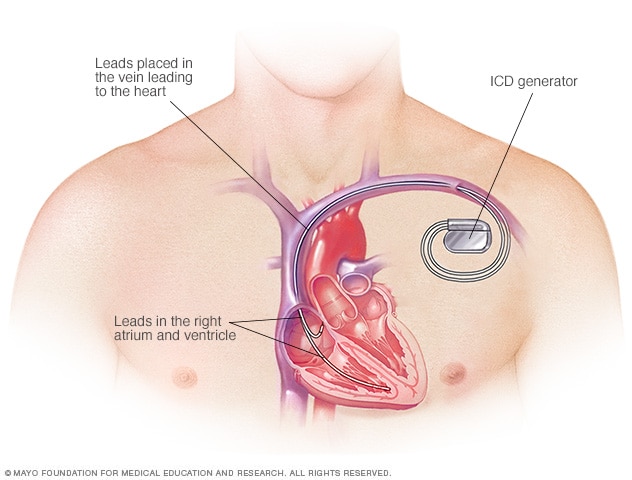
An ICD is surgically placed under your skin, usually below your left collarbone. One or more flexible, insulated wires (leads) run from the ICD through your veins to your heart.
Because the ICD constantly monitors for abnormal heart rhythms and instantly tries to correct them, it helps treat cardiac arrest, even when you are far from the nearest hospital.
How an ICD works
When you have a rapid heartbeat, the wires from your heart to the device transmit signals to the ICD, which sends electrical pulses to regulate your heartbeat. Depending on the problem with your heartbeat, your ICD could be programmed for the following therapies:
- Low-energy pacing therapy. You may feel nothing or a painless fluttering in your chest when your ICD responds to mild disruptions in your heartbeat.
- Cardioversion therapy. A higher energy shock is delivered for a more serious heart rhythm problem. It may feel as if you're being thumped in the chest.
- Defibrillation therapy. This is the strongest form of electrical therapy used to restore a normal heartbeat. During this therapy, it may feel as if you're being kicked in the chest, and it might knock you off your feet.
The pain from this therapy usually lasts only a second. There should be no discomfort after the shock ends.
Usually, only one shock is needed to restore a normal heartbeat. Sometimes, however, you might have two or more shocks during a 24-hour period. Frequent shocks in a short time period are known as ICD storms. If you have ICD storms, you should seek emergency care to see if your ICD is working properly or if you have a problem that's making your heart beat abnormally.
If necessary, the ICD can be adjusted to reduce the number and frequency of shocks. You may need additional medications to make your heart beat regularly and decrease the chance of an ICD storm.
An ICD can also be programmed to perform other functions, which include:
- Antitachycardia (tak-ih-KAHR-dee-uh). If you have an unusually fast heart rate, the ICD delivers painless, low-energy impulses that pace or stimulate the heart to beat normally. This can prevent the need for cardioversion or defibrillation.
- Pacemaker. Most modern ICDs can also function as a pacemaker, delivering low-energy impulses that stimulate the heart to beat normally.
- Recording heart activity. The ICD records information about variations in your heart's electrical activity and rhythm. This information helps your doctor evaluate your heart rhythm problem and, if necessary, reprogram your ICD.
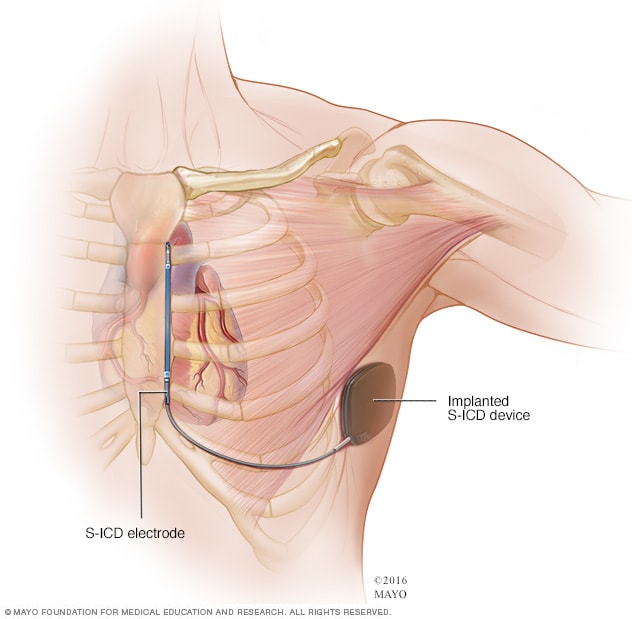
Subcutaneous ICD
A subcutaneous ICD (S-ICD), a newer type of ICD, is available at some surgical centers. An S-ICD is implanted under the skin at the side of the chest below the armpit. It's attached to an electrode that runs along your breastbone. You may be a candidate for this device if you have structural defects in your heart that prevent attaching wires to the heart your blood vessels, or if you have other reasons for wanting to avoid traditional ICDs.
Implanting a subcutaneous ICD is less invasive than an ICD that attaches to the heart, but the device is larger in size than an ICD.
Who needs an ICD
You're a candidate for an ICD if you've had sustained ventricular tachycardia, survived a cardiac arrest, or fainted from a ventricular arrhythmia. You might also benefit from an ICD if you have:
- A history of coronary artery disease and heart attack that has weakened your heart.
- A heart condition that involves abnormal heart muscle, such as enlarged (dilated cardiomyopathy) or thickened (hypertrophic cardiomyopathy) heart muscle.
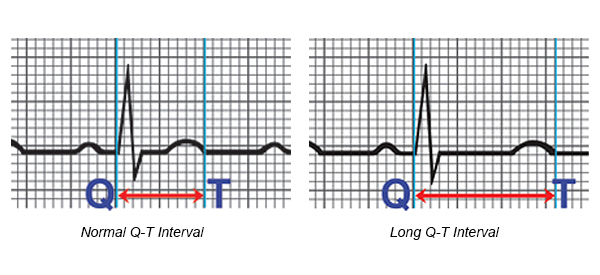
- An inherited heart defect that makes your heart beat abnormally. These include long QT syndrome, which can cause ventricular fibrillation and death even in young people with no signs or symptoms of heart problems.
- Having other rare conditions such as Brugada syndrome and arrhythmogenic right ventricular dysplasia also may mean you need an ICD.
Risks
Risks associated with ICD implantation are uncommon but may include:
- Infection at the implant site
- Allergic reaction to the medications used during the procedure
- Swelling, bleeding or bruising where your ICD was implanted
- Damage to the vein where your ICD leads are placed
- Bleeding around your heart, which can be life-threatening
- Blood leaking through the heart valve where the ICD lead is placed
- Collapsed lung (pneumothorax)
How you prepare
To determine whether you need an ICD, your doctor might perform a variety of diagnostic tests, which may include:
- Electrocardiography (ECG). In this noninvasive test, sensor pads with wires attached (electrodes) are placed on your chest and sometimes limbs to measure your heart's electrical impulses. Your heart's beating pattern can offer clues to the type of irregular heartbeat you have.
- Echocardiography. This noninvasive test uses harmless sound waves that allow your doctor to see your heart without making an incision. During the procedure, a small, plastic instrument called a transducer is placed on your chest.
It collects reflected sound waves (echoes) from your heart and transmits them to a machine that uses the sound wave patterns to compose images of your beating heart on a monitor. These images show how well your heart is functioning, and recorded images allow your doctor to measure the size and thickness of your heart muscle.
- Holter monitoring. Also known as an ambulatory electrocardiogram monitor, a Holter monitor records your heart rhythm for 24 hours. Wires from electrodes on your chest go to a battery-operated recording device carried in your pocket or worn on a belt or shoulder strap.
While wearing the monitor, you'll keep a diary of your activities and symptoms. Your doctor will compare the diary with the electrical recordings and try to figure out the cause of your symptoms.
- Event recorder. Your doctor might ask you to wear a pager-sized device that records your heart activity for more than 24 hours. Unlike a Holter monitor, it doesn't operate continuously — you turn it on when you feel your heart is beating abnormally.
- Electrophysiology study (EPS). Electrodes are guided through blood vessels to your heart and used to test the function of your heart's electrical system. This can identify whether you have or might develop heart rhythm problems.
It's likely you'll be asked not to eat or drink for at least eight hours before your surgery. Talk to your doctor about the medications you take and whether you should continue to take them before your procedure to implant an ICD.
What you can expect
During the procedure
Usually, the procedure to implant an ICD can be performed with numbing medication and a sedative that relaxes you but allows you to remain aware of your surroundings. In some cases, general anesthesia, which puts you to sleep, may be used.
The procedure usually takes a few hours. During surgery, one or more flexible, insulated wires (leads) are inserted into veins near your collarbone and guided, with the help of X-ray images, to your heart. The ends of the leads are secured to your heart, while the other ends are attached to the generator, which is usually implanted under the skin beneath your collarbone.
Once the ICD is in place, your doctor will test it and program it for your heart rhythm problem. Testing the ICD might require speeding up your heart and then shocking it back into normal rhythm.
After the procedure
You'll stay in the hospital one or two days, and the ICD might be tested once more before you're discharged. Additional testing of your ICD usually doesn't require surgery.
Treating pain after your procedure
After surgery, you may have some pain in the incision area, which can remain swollen and tender for a few days or weeks. Your doctor might prescribe pain medication. As your pain lessens, you can take nonaspirin pain relievers, such as acetaminophen (Tylenol, others) or ibuprofen (Advil, Motrin IB, others).
Unless your doctor instructs you to do so, don't take pain medication containing aspirin because it can increase bleeding risk.
When you're released from the hospital, you'll need to arrange for a ride home because you won't be able to drive for at least a week.
Results
ICDs have become standard treatment for anyone who has survived cardiac arrest, and they're increasingly used in people at high risk of sudden cardiac arrest. An ICD lowers your risk of sudden death from cardiac arrest more than medication alone.
Although the electrical shocks can be unsettling, there is evidence that the ICD is effectively treating your heart rhythm problem and protecting you from sudden death. Talk to your doctor about how to best care for your ICD.
After the procedure, you'll need to take some precautions to avoid injuries and make sure your ICD works properly.
Short-term precautions
You'll likely be able to return to normal activities such as exercise, work and sex soon after you recover from surgery. Follow your doctor's instructions. For four weeks after surgery, your doctor might ask you to refrain from:
- Vigorous above-the-shoulder activities or exercises, including golf, tennis, swimming, bicycling, bowling or vacuuming
- Lifting anything weighing more than 5 pounds
- Playing contact sports
- Strenuous exercise programs
Long-term precautions
Problems with your ICD due to electrical interference are rare. Still, take precautions with the following:
- Cellular phones and other mobile devices. It's safe to talk on a cellphone, but avoid placing your cellphone within 6 inches (about 15 centimeters) of your ICD implantation site when the phone is turned on. Although unlikely, your ICD could mistake a cellphone's signal for a heartbeat and slow your heartbeat, causing symptoms such as sudden fatigue.
- Security systems. After surgery, you'll receive a card that says you have an ICD. Show your card to airport personnel because the ICD may set off airport security alarms.
Also, hand-held metal detectors often contain a magnet that can interfere with your ICD. Limit scanning with a hand-held detector to less than 30 seconds over the site of your ICD or make a request for a manual search.
- Medical equipment. Let doctors, medical technicians and dentists you see know you have an ICD. Some procedures, such as magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and radiofrequency or microwave ablation are not recommended if you have an ICD.
- Power generators. Stand at least 2 feet (0.6 meters) from welding equipment, high-voltage transformers or motor-generator systems. If you work around such equipment, your doctor can arrange a test in your workplace to see if the equipment affects your ICD.
- MP3 player headphones. Although the player itself poses little risk, the headphones may be a problem. Most contain a magnetic substance and can interfere with your ICD. Keep your headphones at least 6 inches (about 15 centimeters) from your ICD.
- Magnets. These might affect your ICD, so it's a good idea to keep magnets at least 6 inches (15 centimeters) from your ICD site.
Devices that pose little or no risk to your ICD include microwave ovens, televisions and remote controls, AM/FM radios, toasters, electric blankets, electric shavers and electric drills, computers, scanners, printers, and GPS devices.
Driving restrictions
If you have an ICD to treat ventricular arrhythmia, driving a vehicle presents a challenge. The combination of arrhythmia and shocks from your ICD can cause fainting, which would be dangerous while you're driving.
The American Heart Association's guidelines advise avoiding driving for one week after ICD placement, but talk to your doctor for specific recommendations. The guidelines discourage driving during the first six months after your procedure if your ICD was implanted due to a previous cardiac arrest or ventricular arrhythmia.
If you have no shocks during this period, you'll likely be able to drive again. But if you then have a shock, with or without fainting, tell your doctor and follow his or her recommendations. In most cases, you'll need to stop driving until you've been shock-free for another six months.
If you have an ICD but have no history of life-threatening arrhythmias, you can usually resume driving within a week after your procedure if you've had no shocks. Discuss your situation with your doctor.
You usually can't get a commercial driver's license if you have an ICD.
Battery life
The lithium battery in your ICD can last up to seven years. The battery will be checked during regular checkups, which should occur every three to six months. When the battery is nearly out of power, your old shock generator is replaced with a new one during a minor outpatient procedure.
ICDs and end-of-life issues
If you have an ICD and become terminally ill, your ICD could deliver unnecessary shocks. It's easy to turn off your ICD, and turning it off may prevent unnecessary suffering.
Talk to your doctor about your wishes. Also talk to family members or another person designated to make medical decisions for you about what you'd like to do in end-of-life care situations.