Introduction
The history of cardiac pacing therapy must be viewed within the broader framework of electro-diagnosis and electro-therapy. Moreover it can be observed that the development of electro-therapy usually preceded the understanding of what was actually occurring within the heart.
Electro-therapy has a simple core concept: the use of an outside source of electricity to stimulate human tissue in various ways to produce a beneficial therapeutic effect. This has shown a prolonged, halting development through the ages, sometimes being looked upon as mysterious magic produced by complex machines.
Over the last fifty years or so, electro-therapy has shown a very rapid, almost explosive, development with many innovators contributing to a whole series of “firsts”. This was the consequence of a remarkable co-operation among surgeons, physicians, engineers, chemists, businessmen and patients. The field of paediatric open heart surgery gave a major impetus to the development of pacemakers since heart block often accompanied impeccably performed intra-cardiac repairs of congenital defects.
Most of the scientists and physicians involved in electro-therapy faced significant criticism and sometimes even derision by the contemporary scientific community. Yet the speciality moved steadily on gaining medical respectability and now helps countless patients all over the world.
Hippocrates (460 – 375 BC) (Fig. 1)
“Those who suffer from frequent and strong faints without any manifest cause die suddenly”
Aristotle (384 – 322 BC) (Fig. 2)
Aristotle saw the heart as “the source of all movement, since the heart links the soul with the organs of life”. Remarkably accurite as a description of cardiovascular physiology!
The pulse
In ancient China (280 BC), Wang Shu-he wrote 10 books about the pulse. The Greeks called the pulse “sphygmos”, and sphygmology thus deals with the theory of pulse. Galen, in Roman times, interpreted the various types of pulses according to current thought, that each organ in each disease has its own form of pulse .
Arabian Science age
Ancient Roman physicians
These treated patients suffering from pain and acute gout with electric rays (Fig. 3) and other electrically-charged sea creatures. Electric rays are cartilaginous fish with two large, kidney-shaped electric organs on either side of their head. These organs are capable of generating strong electric shocks, which are administered at will to deter predators or capture prey.
1580
Geronimo Mercuriale (Fig. 4) formulated the concept of syncope and demonstrated its connection with a slow pulse rate: “Ubi pulsus sit rarus semper expectanda est syncope”.
1600
William Harvey restarted an arrested pigeon's heart by a simple flick of the finger. In 1628 he described the circulation (Fig. 5).
1713
While the anatomy and physiology of the heart were being studied, others analysed the peripheral pulse, which was the mechanical expression of cardiac activity. In 1713, Micheal Bernhard Valentini used pulse diagrams and pulse theory in the general practice of medicine (Fig. 6).
Early cardiac electrotherapy
1640's
Publications speculating on the bio-electric nature of the cardiovascular system appeared.
1774
The first reference to external electrical stimulation of the heart in the Registers of the Royal Human Society of London. The physician was Squires and the patient was a young girl.
1775
The Danish physicist Nickolev Abildgaard conducted the first studies on the effects of electrical energy when applied to the body. He placed electrodes on the sides of a hen's head and applied an electric discharge which caused it to fall dead. Application of electrodes over various parts of the hen's body failed to reanimate the bird, until they were placed across the chest. In this position they presumably defibrillated the heart after which the hen staggered onto its feet and walked away (Fig. 7).
1791
Luigi Galvani (Fig.8), an Italian physician and natural scientist, announced that electricity was inherent in organic tissue. He published the experimental findings of electrical phenomena in frog muscles and frog hearts making a fundamental contribution to modern cardiac electrophysiology.
There was general agreement that electricity had a pronounced effect on the heart (Fig.9, 10).
1797
Alexander von Humboldt found a dead bird in his garden and placed a blade of zinc in the beak and a shaft of silver into the rectum. An electric shock caused the bird to flap its wings and attempt to walk. He also tried the experiment on himself with unpleasant consequences.
The 19th century
Rudimentary forms of electrical stimulation were used by physicians sporadically to treat cardiac disease in numerous ways without any standardisation. The crude technology was however far ahead of the understanding of heart disease and a very wide range of effects were documented.
1800
The Italian physicist, Alessandro Volta (Fig. 11) showed that current electricity could be produced by the contact of dissimilar metals and devised the first electric battery for low-voltage high-current stimulation, the “voltaic pile” (Fig. 12). For the first time, electricity could be produced by means other than through electrostatic machines. He also gave his name to one of the basic physical units of electricity: the Volt, unit of electromotive force.
1800 – 1802
Marie Francois Xavier Bichat (Fig. 13) and Nysten reported experiments on decapitated humans in whom they were able to make hearts beat again using electric current. They had no shortage of experimental material during the French Revolution.
1804
Aldini (1762 - 1834) (Fig. 14), described the alleviation of cardiac syncope through "galvanic energy" utilising animal and cadaver studies.
1855
Rudolph Albert von Kollicker published work on the “action currents” of the heart and showed that a definite electric current was produced with each beat of a frog's heart.
1872
Duchenne de Boulogne (1806 - 1875), successfully resuscitated a child who had drowned by attaching one electrode to a leg while rhythmically tapping the precordium with another electrode.
1882
A golden opportunity for clinical and scientific experimentation arose in 1882. A 46-year old female patient arrived in the clinic of Hugo Von Ziemssen (Fig. 15). She was an unskilled labourer called Catharina Serafin from Upper Silesia in Prussia (Fig. 16). A chest tumour had been excised together with the left anterior part of her thoracic wall thus exposing her heart, which could be seen through a thin layer of skin. Von Ziemssen stimulated her heart using electric current and could change her heart rate at will. The recordings (Fig. 17) clearly show that ventricular activity was being produced by electrical impulses applied to the cardiac surface: extremely interesting but potentially fatal investigations!
Late 1800's
The English doctor John Mac William (Fig. 18) collected and analysed all the scattered data available at that time and laid down the basic concepts of modern pacing accurately identifying many of the treatment's problems.
In 1889 he described the application of electricity across the chest to “excite rhythmic contraction… to stimulate by direct means the action of the heart which has been suddenly enfeebled or arrested in diastole by causes of a temporary or transient character”.
Medicine had its first integrated theory of cardiac pacing yet another 20 to 30 years had to elapse before this theory resulted in effective therapy. In particular it had to await significant further medical discoveries (cardiac structure, physiology and conduction pathways) and technical progress (the electrocardiogram, lab stimulators).
The Gerbezius-Morgagni-Adams-Stokes Syndrome
1717
Marcus Gerbazius (1658 - 1718), a Slovenian physician, described the symptoms of bradycardia due to complete atrio-ventricular block.
1761
The Italian Giovanni Battista Morgagni (1682 - 1771) (Fig. 19), founder of pathological anatomy, provided a clinical description of circulatory arrest and implying a causal relationship between a slow pulse and a syncopal attack.
1827
The Irish surgeon Robert Adams (1791 - 1875) described a patient with repeated apoplectic attacks and a slow pulse. He was the first to realise that cerebral symptoms may be caused by cardiac rhythm disorders.
1846
William Stokes (1804 - 1878) (Fig. 20), another Irishman, described once more the pseudo-apoplectic loss of consciousness and bradycardia in a patient, giving further observations on this condition and a detailed analysis of Adam's case.
1899
Karel Frederik Wenckebach (Fig. 21), a Dutchman, described type I second degree atrio-ventricular block in humans using sphygmographic methods of the radial pulse (the electrocardiogram was not yet in clinical use).
1906
John Hay (Fig. 22) from Liverpool, England, published a case report of type II second degree atrio-ventricular block documenting his findings again without the benefit of electrocardiography. He utilised simultaneous tracings from the radial artery and the jugular venous pulse.
1924
Woldemar Mobitz provided the classification of second degree atrio-ventricular blocks utilising the electrocardiogram, hence documenting Wenckebach's and Hay's findings.
The Electrocardiograph
Over the late 1800's – early 1900's, cardiology witnessed a great technological breakthrough that was to have a major effect on the understanding of arrhythmias and hence on the development of specific therapy including pacing: the invention of the electrocardiograph.
1887
The physiologist Augustus Desire’ Waller (Fig. 23) working in St. Mary's Hospital, London, recorded the first human surface electrocardiogram using the Lippmann capillary electrometer to deflect a light beam (Fig. 24, 25). Alexander Muirhead may have been the first to record a human electrocardiogram but Waller was the first to do so in a clinico-physiologic setting, publishing reports and acquiring extensive experience.
Waller had learnt that “each beat of the heart gives an electric change, beginning at one end of the organ and ending at the other”. He was convinced that he could measure these “electromotive properties of the heart” from the skin surface and proceeded to do so with the electrometer connected between the left and right hands or between the front and back paws of his pet bulldog, Jimmie (Fig. 26).
The clinical significance of the electrocardiogram (Fig. 27, 28) was not recognised at the time and Waller himself said: “I do not imagine that electrocardiography is likely to find any very extensive use in the hospital. It can at most be of rare and occasional use to afford a record of some rare anomaly of cardiac action.”
He would often use Jimmie as the subject when he demonstrated his method at lectures by dipping his legs in pots of saline, which served as the electrodes (Fig.29). A question was raised at the House of Commons and this “cruel procedure” risked being dealt with by the “Cruelty to Animals Act” of 1876. The scientist countered these objections and remarked: “If my honourable friend had ever paddled in the sea, he will appreciate fully the sensation obtained thereby from this simple pleasurable experience.” (Fig. 30) Jimmie never complained anyway! Waller is said to have been quite informal and loved entertaining and dashing around with the newly invented motor car.
1892
Another physiologist, Willem Einthoven (1860 – 1927) (Fig. 31), shares the honour with Waller of having founded this new diagnostic modality. Einthoven recorded the first human electrocardiogram in Europe on April 11th, 1892 using the Lippmann capillary electrometer. He initially indicated the four observed deflections with the characters A, B, C, D but later adopted the middle characters of the alphabeth: P, Q, R, S and T (Fig. 32).
In 1902, he made the first direct recording of the true human electrocardiogram using a modified string galvanometer (Fig. 33).
An extremely thin and light weight quartz “string” was silvered to reflect a beam of light and suspended vertically in a strong magnetic field. The very small electric currents generated by the heart were collected from the arms and legs and conducted to the “string” which was deflected laterally by the passage of this fluctuating current. The “string” threw a a vertical shadow, magnified by a microscope, onto a metal plate in which there is a horizontal slot. This slot allows only a point of the shadow to pass through to a moving photographic plate or film, on which the point of shadow writes in a continuous curve. The photographic material was later developed to produce the image (Fig. 34, 35, 36).
The signals were obtained from the two arms and left leg (modern Lead I). To enhance conduction, hands and foot were bathed in saline solution with the tubs wired to the input of the electrocardiograph (Fig.37, 38). It is interesting to note that the signals were collected from a patient in the University Hospital and transmitted to the physiology lab (quite a good distance away) where the actual recording was made. An early example of telemedicine!
There was wide scepticism by the contemporary scientific community against his methods. But Einthoven continued publishing and in 1913 described the Einthoven triangle as the basis for calculations of electrocardiograms and introduced the bipolar electrode system. Classic rhythms were obtained and published.
Einthoven was formal, methodical and demanded technical perfection. He was keen to apply the modality to clinical problems. In 1924, he was awarded the Nobel Prize for Physiology and Medicine for his electrocardiographic work in developing the string galvanometer.
1933
The extremity bipolar electrode system (the standard electrocardiogram lead system) was expanded in 1933 by F. N. Wilson who introduced the unipolar chest wall electrodes.
1942
E. Goldberger introduced the unipolar amplified (augmented) extremity leads. The 12-lead electrocardiogram as we know it today was now complete!
Several manufacturers to produced commercial versions of the electrocardiograph (Fig. 39). The Cambridge Scientific Instrument Co., headed by Horace Darwin (Charles’ youngest son), produced such a device. The string galvanometer for electrocardiography was superceded by direct writing equipment after the Second World War.
Electrocardiography was of paramount importance in the understanding of cardiac rhythm and hence in the further development of pacing.
Late 1920's – Early 1930's: first pacing machines
Credit for the first external cardiac pacemaker has been shared by two doctors: the Australian anaesthesiologist Mark Lidwell and the American physiologist Albert Hyman. Working independently on opposite sides of the world they developed the first cardiac pacing machines.
1928: Mark Lidwell
Lidwell's device ran on alternating current and required a needle to be inserted into the patient's ventricle. In 1928 he used intermittent electrical stimulation of the heart to save the life of a child born in cardiac arrest. The child apparently recovered completely and survived but not much else is known of Lidwell's efforts. He reported his work to the Third Congress of the Australian Medical Society in 1929.
1932: Albert Hyman
Hyman became interested in reviving the “stopped heart” by means of “intracardial (his term) therapy”. Initially this therapy consisted of the intra-cardiac injection of stimulant drugs such as epinephrine although he soon realised that it was not really the drug that restarted the heart but the needle that set up an action current of injury as it punctured the cardiac wall.
Hyman's device, described in 1932 (Fig.40, 41), was powered by a spring-wound hand-cranked motor and called by Hyman himself an “artificial pacemaker”, a term still in current use.
The clockwork drove a DC current generator whose electrical impulses were directed into the patient's right atrium through a bipolar needle electrode introduced via an intercostal space (Fig. 42, 43). Pacing could be delivered at rates of 30, 60 or 120 per minute. None of the three models of this device build in the 1930's survives today and only two photos can be traced.
Hyman’ work (Fig. 44) was frustrated and eventually derailed by technical problems and the attitude of the times. The medical and social community was not ready for electrostimulation: Hyman's device was roundly dismissed as “gadgetry” that interfered with natural events at best and the work of the devil at worst. He faced considerable opposition, including that of the Journal of the American Medical Association and did not report his experiments.
No one agreed to manufacture it locally although a battery-operated version (lost to history) was eventually manufactured by Siemens-Halske in Germany and their American subsidiary Adlanco (Fig. 45). The Hymanotor, as it was called, was tested but found ineffective and again unfavourably reported upon. During the Second World War, Hyman unsuccessfully urged the US Navy to support his device for use in resuscitation of dying servicemen.
It is important to note that Hyman intended the pacemaker to restore a normal heartbeat in patients whose heart had stopped accidentally or in stillborn infants rather than in those with heart block. In the mid-1930's, the connection between Stokes-Adams disease and pacing had not yet been made.
Early 1950's: first mains-powered portable pacemaker
Mains-powered pacemakers were developed in the early 1950's and were large bulky boxes filled with vacuum tubes that could not of course be implanted. They had to be wheeled around on carts and plugged into wall mains socket outlets to obtain their alternating current power. They were portable only in name since they could only go as far as the nearest electrical outlet!
1949
In Toronto, Canada, Wilfred Bigelow (Fig. 46) and John Callaghan started using hypothermia to reduce metabolism and produce bradycardia and asystole to permit cardiac surgery. Re-warming could not however restore cardiac contraction sufficiently rapidly and so the surgeons started experiments with sino-atrial node stimulation.
In 1949, during an experimental operation on a dog, the heart suddenly stopped. Bigelow recounts: “Out of interest and in desperation, I gave the left ventricle a good poke with a probe I was holding. All four chambers of the heart responded. Further pokes clearly indicated that the heart was beating normally with good blood pressure.” The electric pacemaker was developed as a direct result of these hypothermia experiments.
During the 1940's and early 50's the principle device available to generate a variety of electrical impulses, potentially capable of stimulating the heart was a physiological stimulator manufactured by Grass Manufacturing Co for clinical and physiology lab application (Fig. 47). It used a thyratron rectifier tube to convert alternating current into direct current suitable for stimulation of biologic tissue. The stimulation rate, voltage output and pulse width could be varied (monophasic rectangular pulse of 2-20 ms duration).
John Hopps (Fig. 48), an electrical engineer, was recruited on a part-time basis by the National Research Council of Canada and designed what was perhaps the first electronic device specifically built as a cardiac pacemaker. It was an external unit driven by vacuum tubes. The electrical impulses were transmitted via a bipolar catheter electrode to the atria using a transvenous approach. Atrial pacing was readily achieved and heart rate could be controlled with no uncomfortable chest wall contractions (Fig. 49).
1951
Paul Zoll (Fig. 50), a Boston cardiologist, is given credit for ushering in the modern era of clinical cardiac pacing. He had read the work done by Callaghan, Bigelow and Hopps and developed an external tabletop pacemaker that was successfully applied to the treatment of heart block (Fig. 51).
The electrodyne PM-65 pacemaker, designed by Zoll, comprised an electrocardiograph to monitor the cardiac rhythm and an electric pulse generator to pace the heart. The pulse generator was a modification of the electric stimulator used in physiology laboratories. It delivered periodic electric impulses at 2 ms pulse width and 50 to 150 volts alternating current pulse amplitude through a pair of 3 cm2 metal electrodes strapped to the patient's chest directly over the heart. The electrodes irritated the skin and the patients of course found the repeated electric shocks painful.
The mains-powered unit was bulky and heavy and was carried on a cart. It could only go as far as the extension cord would allow (Fig. 52).
In 1952, he reported on two patients suffering from recurring prolonged ventricular standstill whom he treated with this external device (Fig. 53) and in 1956, he applied transthoracic electric shocks to reverse ventricular fibrillation in humans and soon after developed the first cardiac monitors for clinical use.
1956
In St. George's Hospital, London, Aubrey Leatham (Fig. 54) and Geoffrey Davies developed an external stimulator with which to resuscitate patients with heart block and asystole. The first studies on the use of external pacing for cardiac standstill had just been reported by Paul Zoll in Boston; however, Zoll's pacemaker was a fixed system without a demand capability and could cause an “R on T”-induced ventricular fibrillation. At St. George's, Leatham asked Davies to develop the first demand circuit device which was published in 1956.
This mains-powered device stimulated the heart through the intact chest wall utilising 150 volts and was commercially manufactured by Firth-Cleveland in the UK. The commercial version contained several modifications: duration of asystole permitted, sensitivity controls to sense the electrocardiogram, two output ranges and a battery for independent operation.
Late 1950's – early 1960's: The “Golden Years”
These years witnessed several important achievements in the field of cardiac pacing by multiple persons and their teams working in different parts of the world: they were the “golden years” of pacing.
Three landmark “firsts” will be described in detail: the first battery-operated wearable pacemaker (1957), the first totally implantable pacemaker (1958) and the first long-term correction of heart block with a self-contained, implantable pacemaker (1960). These events had far-reaching consequences and opened up the field to the future.
1957: First battery-operated wearable pacemaker
Earl E. Bakken, electrical engineer, TV repairman and co-founder of Medtronic Inc. produced the first battery-operated wearable pacemaker.
The engineer
Earl E. Bakken (Fig. 55) and his brother-in-law Palmer Hermundslie had co-founded Medtronic on April 29th, 1949 in a garage in northeast Minneapolis (Fig. 56, 57). The company had led a precarious existence as a repair service for hospital electrical equipment and regional distributor for other manufacturers. They would build new equipment on order or customise standard instruments for laboratory or clinical researchers. They would hang around hospital surgical suites setting up equipment, training personnel in its use and troubleshooting and repairing it as necessary. Meanwhile they forged working relationships with physicians and their staff.
Bakken recounts: “We never made any serious money on that early custom-building activity, rarely even recouping the cost of the prototype… everything we did, we lost money on!” They were however present at the right time in the right place!
The surgeon
C. Walton Lillehei (Fig. 58) was a leading cardiac surgeon at the University of Minnesota, Minneapolis and had attained international fame by the mid-50's. Techniques had been developed to enter the heart and correct congenital defects while the circulation was supported. By 1957, Lillehei had performed over 300 open-heart operations on young adults and children. This rapidly evolving field of open heart surgery was to be a major driving force towards the development of cardiac pacing.
A series of problems… resolved!
Despite successful repair of the congenital defect, about 1 patient in 10 developed post-operative complete heart block due to damage of the conducting system while the surgical repair was being performed. Stimulant drugs such as adrenaline, atropine, or the newly developed isoprenaline, were helpful in the short-term but proved disappointing over a longer time frame and could not prevent sudden recurrence of heart block. Another solution had to be found!
It was thought that temporary rhythm support via pacing would keep the patient alive until recovery of the conducting system occurred. The technology developed by Zoll was clearly inappropriate as the high voltage pacing stimuli delivered trans-thoracically would be far too traumatic on these young children.
The physiologist John Johnson proposed the utilisation of the Grass stimulator that was used in the physiology labs to activate hearts. After several experiments, Vincent Gott and William Weirich concluded that a cardiac rhythm could be restored in animal hearts in which heart block had been surgically created by means of a wire inserted into the wall of the right ventricle and connected to the external stimulator. Low voltage pulses at the desired rate could easily stimulate these hearts.
Lillehei and his co-workers developed the myocardial wire: a multi-stranded, braided stainless steel wire in a Teflon sleeve (Fig. 59). One end of this was implanted directly into the myocardium and the other end was exteriorised via a stab incision and connected to the physiology lab stimulator. An indifferent electrode was buried under the skin to complete the circuit. Effective pacing needed only 1.5 volts as there was direct contact with the myocardium. There was no rejection and no damage to the beating heart and the wire could be removed easily by tugging once normal conduction resumed.
The first myocardial wire was implanted on the 30th January 1957 in a 3-year old girl in whom heart block had complicated the repair of Fallot's tetralogy. Pacing was successful and the little girl soon regained sinus rhythm and survived. Myocardial wires started being implanted electively, ready for immediate use later should this become necessary. A technique for their implantation through a hollow needle was also developed for non-surgical patients who developed Stokes-Adams attacks.
Further problems soon became obvious: the stimulator was large and heavy, of limited portability and awe-inspiring especially for paediatric patients. Moreover, the system was fatally flawed since it depended totally on its external mains power supply and on the length and integrity of the extension power cord. If power supply failed, it was worthless.
On October 31st, 1957 a municipal power failure lasting three hours resulted in the tragic death of a baby. The hospital had emergency power generation in its surgical suites and recovery area but not in its patient rooms. The caregivers were once more reminded of the limitations of existing technology.
The day after, Lillehei requested Bakken to see if Medtronic could come up with something better. Patient mobility needed improvement and concerns about power failure needed to be eliminated. When Bakken accepted Lillehei's assignment, it seemed to him just like any other special order for a piece of equipment. This was not to be!
First attempts
Initial attempts at building a more reliable and portable pacemaker involved adding an automobile battery with an inverter to convert 6 volts direct current into 115 volts alternating current and then power the conventional alternating current pacemaker on its wheeled stand.
These plans were soon abandoned, as they were obviously highly inefficient! A 10 volt direct current pulse was sufficient to stimulate the heart and transistors were becoming widely available.
The prototype
Bakken dug out the April 1956 back issue of Popular Electronics in which he recalled seeing a circuit for an electronic, transistorised metronome. The circuit transmitted clicks through a loudspeaker: the rate of the clicks could be adjusted to fit the music. The blocking oscillator circuit that was utilised had actually been invented at the MIT Radiation Laboratory during World War II.
He simply modified the two-transistor circuit (Fig. 60) and placed it, without the loudspeaker, into a four-inch-square and inch-and-a-half-thick aluminium box with terminals and switches on the outside. The circuit was powered by a powerful miniature 9.4 volt mercury battery housed within the box. There was an on-off switch and control knobs for stimulus rate and amplitude (Fig. 61).
Bakken recounts: “Without any grandiose expectations for the device, I was moderately optimistic about what it might eventually do for Lillehei's patients. I drove the device over to the University's animal lab where it could be tested on a dog. Of course it worked.
“The next day I returned to the hospital to work on another project when I happened to walk past a recovery room and spotted one of Lillehei's patients. I must have done a double take when I glanced through the door. The little girl was wearing the prototype I had delivered only the day before! I was stunned. I quickly tracked down Lillehei and asked him what was going on. In his typical calm, measured, no-nonsense fashion he explained that he’d been told by the lab the pacemaker worked and he didn’t want to waste another minute without it. He said he wouldn’t allow a child to die because we hadn’t used the best technology available.”
After only 4 weeks of experimentation and work, the first battery-powered, transistorised pacemaker was already in clinical use! A feat that is unlikely ever to be repeated given the regulatory labyrinth that all devices have to go through from inception to clinical use.
The “first ten”
The first production run of ten or so units were more refined versions of the original prototype and went into clinical use soon after at the University (Fig. 62). The dials had been recessed so that children would be less likely to adjust them and a little neon light blinked red with each stimulus. In addition, two metal handles (borrowed from an old ECG machine) were been added such that a strap could secure the pacemaker to the body. The pacemaker was not only portable but wearable! This pacemaker became known as the 5800 (because it was made in 1958). The product literature (Fig. 63) stated boldly:
“So small and light that it may be attached to and worn by the patient, the Medtronic Cardiac Pacemaker stimulates ventricular function in cases of atrio-ventricular dissociation that are induced during the surgical repair of septal defects, or that occur spontaneously as in Stokes-Adams syndrome. The Pacemaker is designed for internal applications with at least one wire attached directly to the myocardium for temporary stimulation or with a bipolar patch for prolonged stimulation.
“Created with imagination and originality, the transistorised circuit completely removes the hazards and nuisance associated with AC powered instruments. Its self-contained miniature power source will operate the instrument for approximately 1000 hours.”
The chosen pacemaker output was a 2 ms square wave, variable in amplitude from 1 to 20 mA into a 1000 Ω load. The blocking oscillator repetition rate was variable from 60 to 180 pulses per minute. The box was “wearable” (Fig. 64).
The significance and the consequences
Medical historians regard Bakken's pacemaker is one of the first successful applications of transistor technology to medical devices helping to launch the new field of “medical electronics” (Fig.65). In the entire history of medicine before 1957, there had never been a partly or completely implantable electrical device. It was however apparent that for long-term pacing a totally implanted device would have to be designed as ascending infection via the pacing electrodes occurred frequently.
Lillehei himself noted: “The question of how long stimulation can be maintained appears to be related to electrode materials, design and technique of implantation… The possibility of infection along the wire exists, but… can be minimised by tunneling the wire for some distance before bringing it out on through the skin.” (Fig. 66) Most patients with post-operative heart block regained sinus rhythm within a few weeks but one patient was kept on the device for 15 months.
Recurrent heart block in patients who had recovered from their post-operative heart block caused several deaths. It was apparent that these patients needed indefinite and not temporary pacing for them to survive. The myocardial wire developed exit block as scar tissue grew around the site of stimulation increasing electrical resistance and requiring a progressive increase in pacing stimulus voltage to maintain capture. The thoracic muscles began to twitch at these increased voltages. A totally implantable system with better designed elctrodes neede to be designed!
Meanwhile elsewhere, on the 16th July 1958 a transvenous catheter electrode was introduced fluoroscopically, via the basilic vein into the right ventricular outflow tract, in a patient with fixed complete heart block who required colon resection because of a malignancy. Pacing was continued for two hours, during the operative procedure, and ended with slowing of the stimulation rate until an unpaced idioventricular rhythm developed. The catheter was removed without complication and the patient resumed the idiventricular bradycardia.
1958: First implantable pacemaker
On October 8th, 1958 the first pacemaker implantation was performed in Sweden. The system had been developed by the surgeon Ake Senning and the physician inventor Rune Elmqvist and implanted on a 43-year old engineer called Arne Larsson. This first experience with a fully implantable pacemaker system was reported at the Second International Conference on Medical electronics in 1959 and published as an abstract in 1960 (Fig. 67).
The patient suffered from Stokes-Adams attacks that required resuscitation many times daily and whose situation was considered hopeless. The implantation was a more or less desperate rescue measure. The risks taken with this completely unknown therapy were immense.
The scientists
Ake Senning (Fig. 68) was the cardiac surgeon in charge of the Department of Thoracic Surgery at the Karolinska Hospital in Stockholm. He had observed Lillehei's work with temporary external pacing.
Rune Elmqvist (Fig. 69) was a medical graduate who had not pursued a medical practice but became an engineer. He had designed a portable ECG machine in 1931 and then the widely used ink jet recorder, the Mingograf, in 1948.
These two men began to collaborate closely in 1950 and developed fibrillators and defibrillators for open heart surgery. They realised that the main problem with external pacemakers was the open route for ascending infection along the lead and decided to design a fully implantable system.
The patient
Arne Larsson (Fig. 70) is the first human to receive an implanted pacemaker. He had been hospitalised with complete heart block and frequent Stokes-Adams attacks for 6 months. He was having 20 to 30 attacks daily and his prognosis was poor. Treatment was maximised with ephedrine, pentymal, atropine, isoprenaline, caffeine, digoxin and whisky.
The woman: Else Marie Larsson
Else Marie was the patient's wife who pleaded with Elmqvist and Senning to help her hopelessly ill husband. She had read press reports about ongoing experiments with electrical stimulation of the heart and hounded down the two scientists for a solution that did not yet exist: an implantable pacemaker.
Senning recounts his encounter with this lady: "An energetic, beautiful woman entered my lab on the 6th October 1958 and told me that I had to implant a pacemaker into her husband. I told her we had not completed our experimental series and we did not have a pacemaker for human clinical implantation. She demanded: ‘So make one!’. That day she drove several times from Elmquist's electronic lab and back and finally convinced us.”
The procedure
To avoid publicity, the implantation was done in the evening when the operating rooms were empty. Via a left-sided thoracotomy two suture electrodes were implanted into the myocardium and tunnelled to the pacemaker box placed in the abdominal wall. The first pacemaker implanted functioned only a few hours but the second one implanted in the same patient had better longevity.
Senning recounts: “On the 8th October 1958, in the evening, when there were no extra people in the theatre, I implanted the first pacemaker, but it lasted only 8 hours. Presumably, I had damaged the output transistor or capacitance with the catheter and I did not have the other one which was in the lab. I implanted the other one early the next morning”. Senning then concludes: “In the 1950's we did not have any liability problems. The patient and relatives were happy if the patient survived.”
The second pacemaker functioned well for about 1 week before suddenly showing a decrease in the ECG pacing stimulus size: suggesting probable lead fracture rather than pulse generator malfunction.
The circuit
The pulse generator delivered impulses at an amplitude of 2 volts and a pulse width of 1.5 ms. The pulse rate was fixed at a constant rate of 70 to 80 beats per minute. The energy utilised was minimised since Elmqvist managed to obtain a few of the first silicon transistors imported into Sweden. These were more efficient than the older germanium transistors. With them Elmqvist designed a stable and efficient blocking oscillator with a small power consumption (Fig. 71).
The first transistor forms a repetitive blocking oscillator whose pulses are fed to the base of the second transistor. the collector of this second transistor is then connected to the pacing electrode over an RC network.
Several types of primary battery cells could have been used. The Ruben-Mallory cells with zinc as the anode and mercuric-oxide as the depolarizer were a possible choice (Fig. 72). They had been invented during World War II for army field telephones. Although the cell potential remained constant, these cells had a short lifetime and released hydrogen gas at the zinc anode. The effect of this gas in a cell encapsulated in plastic was not known. For these reasons, nickel-cadmium rechargeable cells were then chosen. Two cells of 60 mAh each were sealed, encapsulated and connected in series.
Recharging was accomplished inductively. A coil antenna with a diameter of about 50 mm was connected to the cells via a silicon diode. This was inductively coupled across the patient's skin to a large external flexible coil 25 cm in diameter attached to the patient's abdomen with adhesive tape. Recharging was accomplished by a 150 kHz radio frequency current generated by an external mains-powered vacuum tube device connected to the external coil. The pacemaker required charging once a week for 12 hours.
The device
The entire unit was entirely hand-made (Fig. 73) and consisted of the nickel-cadmium batteries, the electronic circuit and the coil recharging antenna. These were encapsulated in a new epoxy resin (Araldite) produced by Ciba-Geigy, which had excellent biocompatibility. The approximate diameter and thickness became 55 mm and 16 mm respectively, according to the dimensions of the ever so popular shoe polish can from Kiwi (Fig. 74). Elmquist in fact produced two such units using these cans as moulds!
These first units had two electrode wires, each consisting of a twinned, stainless steel suture wire with polyethylene insulation. The distal ends of the wires were sewn into the myocardium to act as pacing electrodes. The proximal ends were hard-wired to the pulse generator circuit. It was estimated that the electrode had to withstand about 105 bends per day (Fig. 75, 76).
The personal outcome
Rune Elmqvist soon ceased his involvement in pacing but remained active in other areas of medical technology. He died in 1997, aged 90. Ake Senning remained very active in the field of cardiac surgery. He died in 2000 at the age of 84. Arne Larsson survived both the engineer as well as the surgeon who had saved his life (Fig. 77). He required five lead systems and 22 pulse generators of 11 different models until his death on December 28th 2001 aged 86 of a malignancy totally unrelated to his conduction tissue disease or his pacemaker system.
1960: The first long-term correction of complete heart block
Wilson Greatbatch
Wilson Greatbatch was an electrical engineer teaching at the University of Buffalo where he was working on an oscillator to aid in the recording of tachycardias. He accidentally discovered the way to make an implantable pacemaker (Fig. 78).
Greatbatch, a deeply religious man, describes the event this way: “It was no accident, the Lord was working through me… The oscillator required a 10 KΩ resistor at the transistor base. I reached into my resistor box for one, but I misread the color coding and got a 1 MΩ resistor by mistake.”
When he plugged in the resistor, the circuit started to “squeg” with a 1.8 millisecond pulse followed by a 1 second interval during which the transistor was cut off and drew practically no current. “I stared at the thing in disbelief,” he said. Wilson Greatbatch immediately realized that this small device could drive a human heart but it wasn’t easy to find a heart surgeon who would believe in his idea.
Dr. William Chardack
Dr. William Chardack was chief of surgery at Buffalo's Veteran's Hospital at the time. In Dr. Chardack, Greatbatch had finally found a surgeon who believed in the viability of an implantable pacemaker.
On May 7, 1958, Greatbatch brought what would become the world's first implantable pacemaker to the animal lab at the hospital. There, Chardack and another surgeon, Dr. Andrew Gage, exposed the heart of a dog to which they touched the two pacing wires. The heart proceeded to beat in synchrony with the device. The three looked at each other. Their feelings were best expressed by Dr. Chardack, who exclaimed, “Well, I’ll be damned.”
In a lab book about a year later, Greatbatch wrote “I seriously doubt if anything I ever do will give me the elation I felt that day when a 2 cubic inch electronic device of my own design controlled a living heart.”
The “bow tie team” (Fig. 79)
The three - Greatbatch and Drs. Chardack and Gage - became known as the bow tie team. “The two doctors wore bow ties because children tend to pull long ties. I wear bow ties because long ties get in the way when I am soldering.” The months and years that followed involved a great deal of research and experimentation. “I frequently took problems to the Lord in prayer,” Greatbatch says, “and I always got the answer.”
Over the first two years experiments were made with animals. In 1959, Greatbatch patented the implantable pacemaker, and William Chardack reported the first success in a human with this unit in 1960. The procedure was completed in June 1960 on a 77-year old man in complete heart block (Fig. 80). Chardack first implanted the lead and when threshold stabilised implanted the pulse generator. The patient survived uneventfully for 2 years before his death from natural causes.
In 1961, Chardack, Gage and Greatbatch reported a series of 15 patients who had pacemakers implanted. Greatbatch later invented the long-life corrosion-free lithium-iodine battery to power the pacemaker (Fig. 81).
The global outcome
The early pacing technology of the 1950's and 1960's was a spin-off from the research and development of World War II and Cold War eras.
Faulty batteries, body fluids leaking into the encasement and broken leads caused numerous pacemaker failures that required emergency surgery. The main difficulty however was the lead. It was soon obvious that the myocardial wire was unsuitable as a long-term electrode. Stimulation threshold increased after a few weeks until exit block developed and no more capture was possible. Moreover, the wire could not resist the enormous repetitive mechanical stresses of bending. These technical problems contributed to the delay in the widespread use of implanted pacemakers for several years.
Tight collaboration between engineers, physicians and patients was the fundamental driving force for the growth of a significant global industry. Well over 2 million pacemakers have been implanted worldwide since 1960!
Zoll founded Electrodyne and continued developing pacemakers.
Earl Bakken (co-founder of Medtronic Inc.) started producing the Chardiack-Greatbatch pacemaker.
Wilson Greatbatch, after a time with Medtronic, founded his company (Wilson Greatbatch Ltd.) and convinced the industry to change from mercury to lithium-iodine cells.
The company Elema Schonander, for which Rune Elmqvist worked, became Siemens-Elema in 1974. Siemens then acquired Pacesetter Inc. in 1985 and combined them to form Siemens-Pacesetter which was then in turn acquired by St. Jude Medical in 1994
1958-1959: Inductively coupled cardiac pacemakers
Other investigators followed a different line of approach in designing self-contained implantable pacemakers: inductive coupling (Fig. 82).
A pair of electrodes were sutured to the epicardium and connected to a coil antenna located subcutaneously. Minimal or no circuitry was implanted and no internal batteries were needed.
This coil antenna was inductively coupled to an external coil taped to the patient's intact skin. This external coil was connected in turn to a transistorised pulse generator powered by an external battery. The electronic components, relatively unreliable at this time, were therefore located entirely outside the body.
Glenn, Mauro, Longo, Lavietes and Mackay's technique utilised a radio-frequency oscillator . Later versions of this system included triple-helix, silicone insulated endocardial leads and rate-control via an external knob (which the patient himself could modify at will). Atrial pacing with this device was used in 1969.
Inductively-coupled pacemakers proved to be very successful with several hundreds of implants and survival rates of over 10 years (Fig. 83). These devices were extensively used in the Birmingham (UK) region for a number of years, being produced by the Lucas factory, more commonly known for its automotive electrical products (until taken over by Bosch). One particular disadvantage of this device was that its removal (for example, for bathing) could result in bradycardia and syncope. They continued to be used until well into the 1970's and several patients with later generation pacemakers still have the implanted coils from their original devices.
1959: Hunter – Roth electrode
On the 4th April 1959 Samuel Hunter (Professor of Surgery at St. Paul) and Norman Roth (Chief Engineer at Medtronic) implanted a bipolar stainless steel electrode to pace a patient suffering from post-myocardial infarction complete heart block (Fig. 84). The lead consisted of a pair of stainless steel wires secured in a silicone rubber base (Fig. 85).
1959: Elema-Ericsson lead
A new lead was developed in 1959 by Elema Schonander and the Telecom Company, Ericcson. This consisted of four thin bands of stainless steel wound around a core of polyester braid and insulated with soft polyethylene (Fig. 86). It was estimated to resist over 184 million flex cycles, hence lasting for at least 6 years. The unipolar epicardial stimulation electrode was a platinium disc, 8mm in diameter and insulated at the back.
1959 - 1960: Elema Schonander
The Elema 135 (Fig. 87) rechargeable pacemaker was successfully implanted in Stockholm (1959), Uruguay (February 1960) and England (March 1960) but Elema Schonander never filed a patent application. The maket prospects were perceived to be poor! Pacemakers were considered as an expensive service to prominent customers with little commercial value. The external charging system was too complicated especially for elderly patients.
Elmqvist constructed the Elema 137 pacemaker in 1960 (Fig. 88). Ruben-Mallory zinc-mercury oxide cells were used as the power source thus eliminating the need for periodic recharging of the previously utilised nickel-cadmium cells.
Early 1960's
Other models were implanted with similar success in 1961 by Zoll et al (Fig. 89) and in 1962 by Kantrowitz et al. The technique for inserting permanent transvenous bipolar pacing electrodes was developed in 1962 by Parsonnet et al. (in the US) and by Ekstrom et al. (in Sweden).
Recent history
Pacemaker and lead technology continued to develop rapidly to make these devices reliable, automatic and flexible in the therapy they provide. The therapeutic end-point shifted from saving life to enhancing its quality and simplifying follow-up. Electrotherapy has become socially accepted and its indications are extending also to non-cardiac pathology: Parkinson's Disease, pain-control, drug delivery.
Mid 1960's (Fig. 90)
Transvenous leads replaced epicardial leads. Pacemakers and their leads could be implanted without a thoracotomy and without general anaesthesia. “Demand” pacemakers were developed to sense the underlying cardiac activity and provide pacing only when needed.
1970's
Lead design improved: “tined” for passive fixation and “screw-in” for active fixation. The lithium-iodine battery was developed to replace the mercury oxide-zinc battery that had been used till then. This resulted in greatly increased pacemaker longevity (Figs. 91 and 92).
In 1972 an American-made radioisotope pacemaker was implanted by Parsonnet et al. These nuclear pacemakers had an expected life of 20 years but went out of fashion mainly due to the need for extensive regulatory paperwork (Fig. 93).
Titanium casing was developed to enclose the battery and circuitry. This replaced the epoxy resin and silicone rubber that was previously utilised to encase the internal components of the pacemaker.
Pacemakers were made non-invasively programmable in the mid-1970's. Using a radio-frequency telemetry link, most pacing parameters could be adjusted to follow the changing clinical needs of the patient.
By the end of the 70's dual-chamber pacemakers were developed to pace and sense in both atria and ventricles. Synchronised timing made it possible to preserve the atrial contrbution to ventricualar filling as well as to track the intrinsic atrial rate.
1980's
In the early 1980's steroid-eluting leads were developed. These eluted steroid from their tip and hence decreased the inflammatory response evoked by the presence of the lead tip (acting as a foreign body). Consequently, the early rise of capture threshold was blunted and safety was enhanced (Fig. 94).
In 1981, Zoll patented and re-introduced a transcutaneous external pacemaker with a longer pulse width of 40 ms and a larger electrode surface area of 80 cm2. This reduced the current necessary to capture the heart and thus improved patient comfort. This method of pacing could be applied very rapidly as a bridge to a the establishment of pacing via the transvenous route.
In the mid-1980's rate-responsive pacemakers were designed. A tiny sensor within the pacemaker box detected body movement and used this as a surrugate measure of activity. Signals from the sensor were filtered and applied to an algorithm to alter the pacing rate up or down. Thus, pacing rate would change according to the patient's activity level.
1990's
Microprocessor-driven pacemakers appeared. These became very complex devices capable of detecting and storing events utilising several algorithms. They delivered therapy and modified their internal pacing parameters according to the changing needs of the patient in an automatic manner. The rate-response pattern also adjusted itself automatically to the patient's activity level (Fig. 95).
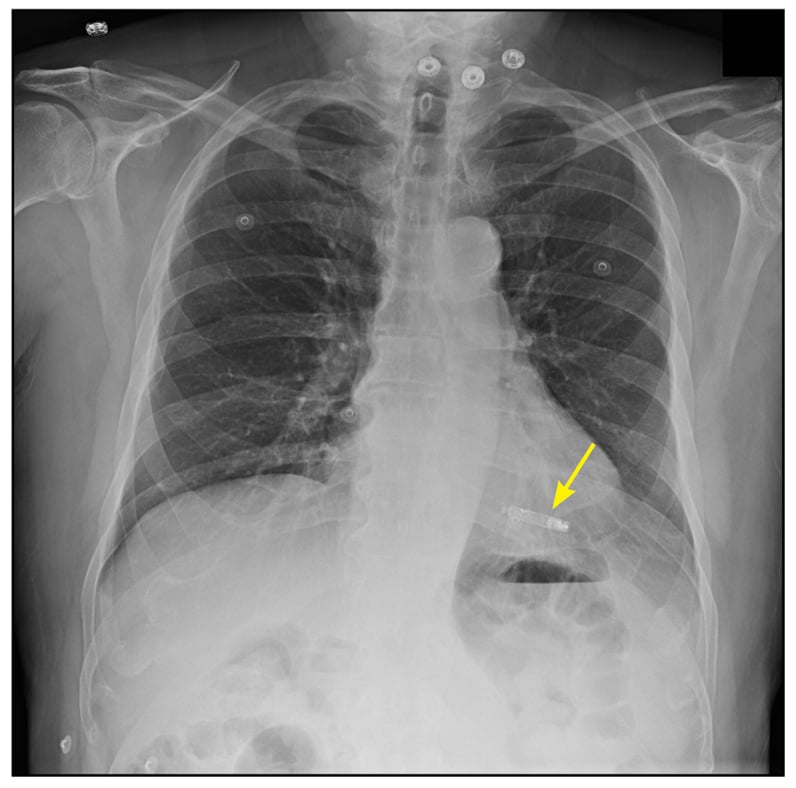
2000's
Bi-ventricular pacing for heart failure was introduced. An additional specially-designed lead was introduced via the coronary sinus to the epicardial surface of the left ventricle. The right ventricle (via the standard lead) and the left ventricle were paced simultaneously to attempt to resynchronise contraction of the left ventricular septum and left ventricular lateral walls. The improved contraction improved symptoms and survival (fig. 96).
Automaticity progressively increased thus making follow-up visits easier and briefer. Pacemakers could also upload data telephonically to a central server via the internet (Fig. 97).