I. Atrial Tachycardia: What every physician needs to know.
An atrial tachycardia is a fast abnormal heart rhythm in which the electrical impulse originates in atrial tissue different than the sinoatrial node. Atrial electrical activation during atrial tachycardias is mostly regular and by definition at a rate faster than 100 bpm, although occasionally the rate may oscillate and be slower.
Atrial tachycardias are the least frequent form of supraventricular tachycardias in the general population. The mechanisms of the arrhythmia could be abnormal automaticity, triggered activity, or reentry that is limited to atrial tissue.
Classification
Atrial tachycardias are a form of supraventricular tachycardia and can be classified as focal or macro-reentrant, depending on their origin and propagation of the electrical impulse. They can also be classified by the mechanism of the arrhythmia. Focal atrial tachycardias are frequently assumed to be due to automaticity although micro-reentry and triggered activity are possible and difficult to demonstrate in practice.
Macro-reentrant atrial tachycardias involve the participation of a reentry circuit within the atria. Neither the compact atrioventricular node (AV) node nor the accessory pathways participate in the mechanism of this arrhythmia. Atrial tachycardias can also be classified by the anatomic atrial structure where they originate from or that is involved in the reentry circuit (i.e., crista terminalis, tricuspid annulus, atrial appendages, mitral annulus-aorta continuity, and scar-related or mitral annulus reentry).
Clinical presentation
The clinical presentation of this arrhythmia varies significantly from short, rather sporadic, paroxysmal runs to incessant tachycardia. Atrial tachycardias are often misdiagnosed as panic attacks and anxiety like any supraventricular tachycardia. The arrhythmia is commonly documented in the surface electrocardiogram as a narrow complex tachycardia and generically diagnosed as a supraventricular tachycardia.
A definitive diagnosis of an atrial tachycardia may be only possible through specific clinical or electrophysiologic features of the arrhythmia requiring electrocardiography and sometimes even intracardiac recordings. Occasionally incessant atrial tachycardias can present as congestive heart failure secondary to dilated cardiomyopathy. The diagnosis of tachycardia-induced cardiomyopathy and adequate treatment of the atrial tachycardia could be life saving and result in complete resolution of the cardiomyopathy. Hemodynamic instability is possible but rather rare during atrial tachycardias.
II. Diagnostic Confirmation: Are you sure your patient has an Atrial Tachycardia?
The diagnostic confirmation of an atrial tachycardia requires detailed analysis of electrocardiographic or intracardiac electrogram recordings. Definitive diagnosis can be suspected but not confirmed in clinical grounds only.
The clinical suspicion of an atrial tachycardia is usually brought by symptoms compatible with a supraventricular tachycardia.
Sudden onset and offset of a regular narrow complex (QRS <120 ms) tachycardia coincidental with the presenting symptoms is very suggestive of a supraventricular tachycardia.
Short bursts of tachycardia in between sustained episodes may suggest the diagnosis of atrial tachycardia but can be seen with any other form of supraventricular tachycardia. During atrial tachycardias, the P–R interval is usually normal, although in the presence of AV nodal disease the P–R interval could be prolonged.
A rare but potentially fatal presentation is tachycardia induced cardiomyopathy with the constellation of symptoms caused by progressive systolic heart failure including edema, dyspnea on exertion, orthopnea and paroxysmal nocturnal dyspnea with documented depressed left ventricular systolic function in the setting of persistent tachycardia.
A. History Part I: Pattern Recognition:
Key symptoms: palpitations, chest discomfort, dyspnea at rest or exertion, dizziness, fatigue, near syncope, rarely syncope or could be asymptomatic.
Key physical signs: regular tachycardia, unusual blood pressure (either higher or lower than baseline), pallor or flushing, rapid vascular peripheral pulsation waves, heart failure signs can be present when the tachycardia is associated with heart failure.
Focal and reentrant atrial tachycardias have overlapping characteristics but may present with different clinical patterns and prevalence among different patient populations. Both can lead to tachycardia-induced cardiomyopathy when incessant over periods of several weeks or months.
Focal atrial tachycardias
Focal atrial tachycardias are rather infrequent and most commonly seen in normal hearts and younger patients, although they could develop at any age. They can present as an incessant tachycardia, which makes the diagnosis easier or as intermittent episodes of palpitations, as any other paroxysmal supraventricular tachycardia of sudden onset and offset. Focal atrial tachycardias can show acceleration and deceleration in response to changes in the autonomic tone with slight rate variations. Their origin has been described from multiple atrial and vascular structures within the atria, including crista terminalis, along the tricuspid and mitral annulus, pulmonary veins, fossa ovalis, coronary sinus, mitral annulus-aorta junction, vein of Marshall and atrial appendages (Figure 1 , Figure 2 ).
Figure 1.
Electrocardiogram of a 6-year-old patient presenting with an incessant atrial tachycardia and tachycardia-induced cardiomyopathy. An electrophysiology study demonstrated a focal atrial tachycardia originating in the anterior rim of the fossa ovalis. Radiofrequency ablation resulted in elimination of the tachycardia and resolution of the cardiomyopathy later on.
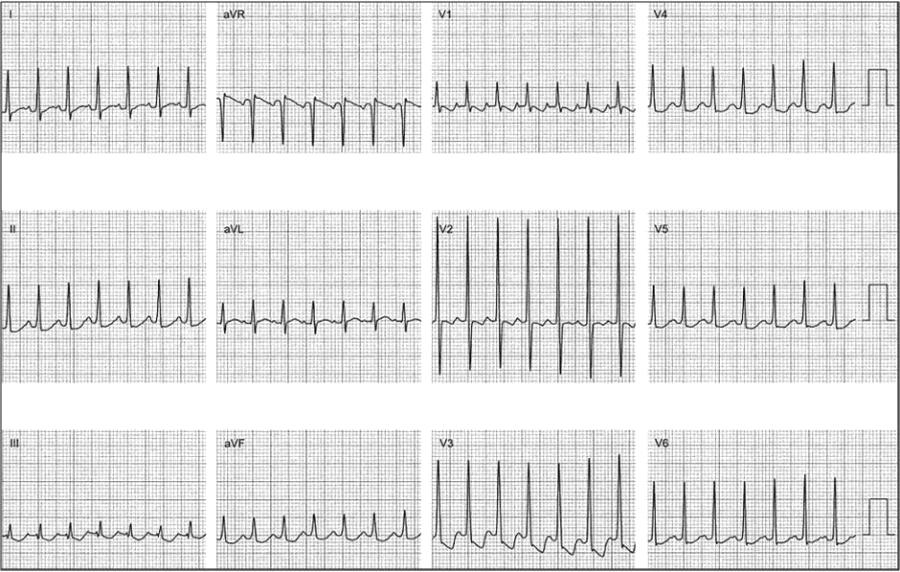
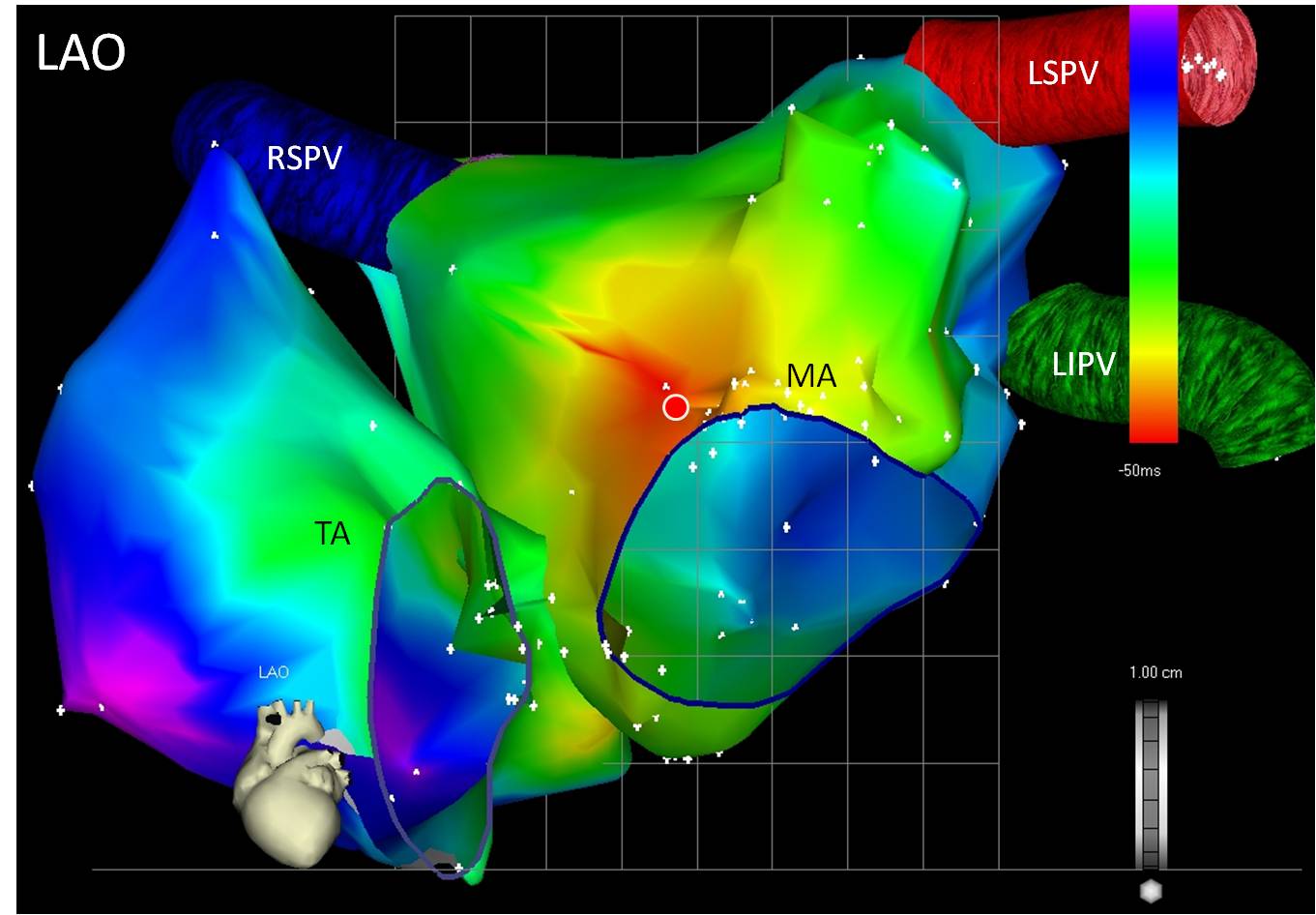
Figure 2.
Three dimensional nonfluoroscopic activation map of a focal atrial tachycardia originating in the mitral annulus-aorta continuity. Both right and left atria are shown with color-coded representation of the timing intervals. The red area represents early activation and the solid circle the earliest area of activation where radiofrequency ablation resulted in termination of the tachycardia. LAO: left anterior oblique. TA: tricuspid annulus. MA: mitral annulus. RSPV: right superior pulmonary vein. LSPV: left superior pulmonary vein. LIPV: left inferior pulmonary vein.
Macroreentrant atrial tachycardias
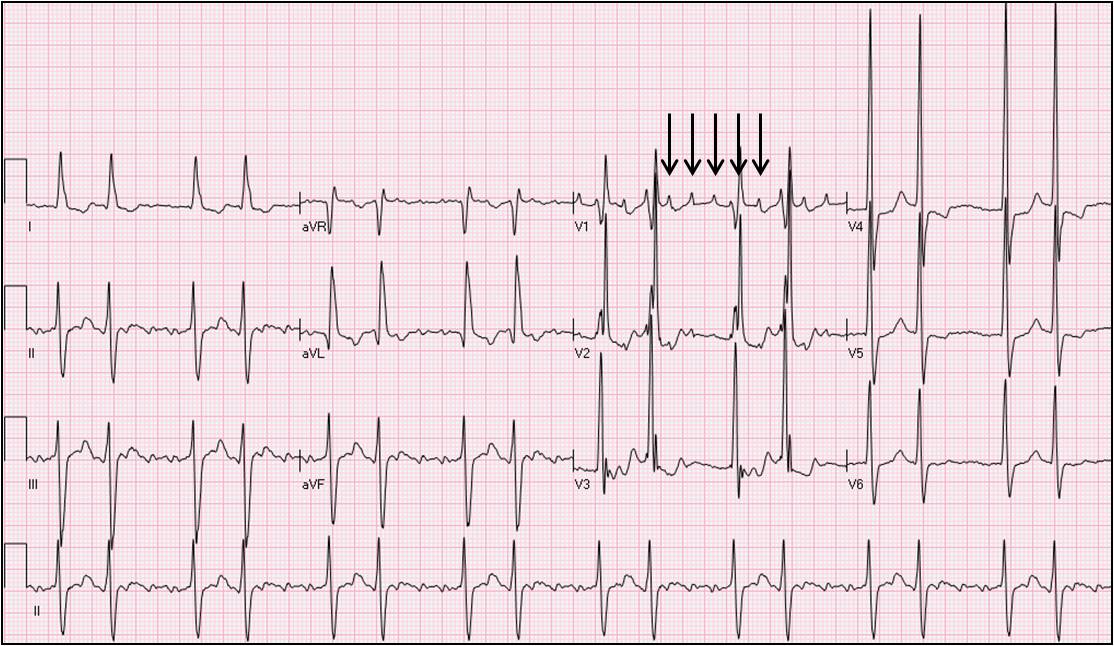
Macro-reentrant atrial tachycardias are more frequent among patients with a history of structural heart disease, prior cardiac surgery, chronic obstructive pulmonary disease, pulmonary hypertension, prior ablation procedures involving the atria, or any other clinical condition leading to atrial dilatation or atrial scaring. Macro-reentrant atrial tachycardias could resemble typical cavo-tricuspid isthmus dependent atrial flutter (using a puristic definition atrial flutter itself could be considered a form of macroreentrant atrial tachycardia) and are frequently persistent although they could be paroxysmal as well (Figure 3, Figure 4).
Figure 3.
Electrocardiogram of a 68-year-old man presenting with an asymptomatic tachycardia. The electrocardiogram is suggestive of an atypical atrial flutter with variable atrioventricular conduction. An electrophysiology study demonstrated a macro-reentrant atrial tachycardia around the mitral annulus with an area of slow conduction medial to the left atrial appendage on the anterior mitral annulus. Solid arrows point to the atrial tachycardia waves in lead V1.
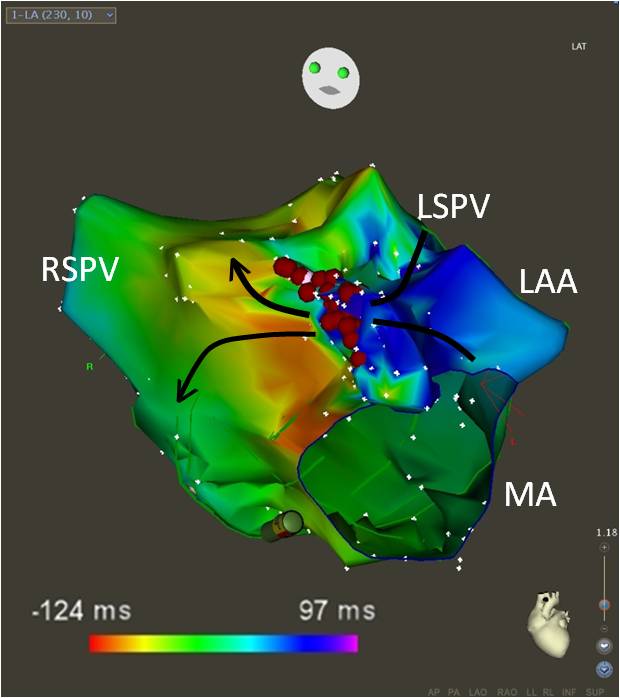 Figure 4.
Figure 4.
Three-dimensional nonfluoroscopic activation map of the macro-reentrant atrial tachycardia depicted in Figure 3. An area of slow conduction on the anterior mitral annulus medial to the left atrial appendage constituted the isthmus of the reentry circuit. Red solid circles correspond to the ablation lesions. The solid arrows indicate the direction of activation of the tachycardia across the isthmus. Color coding corresponds to activation timing. Areas red-yellow correspond to early activation and blue-purple late activation. The anatomic location where early meets late corresponds to the isthmus of the tachycardia. MA: mitral annulus. RSPV: right superior pulmonary vein. LSPV: left superior pulmonary vein. LAA: left atrial appendage.
B. History Part 2: Prevalence:
Atrial tachycardias, excluding atrial flutter, are thought to represent about 5% to 15% of sustained supraventricular tachycardias in the adult, and are more frequent in the pediatric population. Short, self-terminated runs of atrial tachycardia are rather common in ambulatory heart rhythm monitor recordings; but when asymptomatic and sporadic, they are frequently dismissed as incidental findings and not considered a clinical entity.
Atrial tachycardias become more prevalent with age at the expense of a higher representation of macro-reentrant atrial tachycardias in the older population, while focal atrial tachycardias become rather rare. Focal atrial tachycardias are more common among younger patients with normal hearts. A gender difference in the prevalence of these arrhythmias is not clear, although higher prevalence of focal automatic atrial tachycardias in women has been reported.
C. History Part 3: Competing diagnoses that can mimic an Atrial Tachycardia
The other forms of supraventricular tachycardia are very difficult to differentiate from atrial tachycardias from the clinical standpoint:
- Typical AV nodal reentrant tachycardia (slow-fast AVNRT): cannon A waves appreciated at physical exam during tachycardia are suggestive of typical AVNRT. Undistinguishable P waves (simultaneous with the QRS), an RSr' pattern during tachycardia in lead V1 that is absent during normal sinus rhythm, or P waves with a superior axis right after the QRS in the surface electrocardiogram are suggestive of typical AVNRT.
- Atypical AV nodal reentrant tachycardia (fast-slow or slow-slow AVNRT): electrocardiographically very difficult to differentiate from an atrial tachycardia. An electrophysiology study may be necessary to make a definitive diagnosis.
- Atrioventricular reentrant tachycardia (AVRT): during tachycardia, the P waves are usually close to the preceding QRS in AVRT resulting in a rather long P–R interval, which suggests a diagnosis different than atrial tachycardia but still possible in atrial tachycardias with prolonged P–R. An electrophysiology study may be necessary to make a definitive diagnosis.
Permanent junctional reciprocating tachycardia (a unique form of AV reentrant tachycardia using a slow conducting retrograde accessory pathway): because of the participation of a slowly conducting accessory pathway as the retrograde limb of the tachycardia, this arrhythmia has retrograde P waves with a superior axis close to the following QRS resulting in a relatively normal P–R interval and very difficult to differentiate from an atrial tachycardia. The incessant nature of this arrhythmia, younger age of presentation, and frequent presentation as tachycardia-induced cardiomyopathy help lead the clinical differential diagnosis towards this entity.
- Junctional ectopic tachycardia: rather rare and more common in children. As a focal tachycardia originating in AV nodal tissue, it clinically presents similar to focal atrial tachycardias, but the electrocardiographic recordings are similar to those of typical AV nodal reentrant tachycardia usually showing absence of P waves that are simultaneous and obscured by the QRS. An electrophysiology study with pacing maneuvers is necessary to make a definitive diagnosis.
- Ventricular tachycardia: any supraventricular tachycardia that conducts to the ventricle with aberrancy resulting in a wide complex tachycardia, either due to bundle branch block or intraventricular conduction delay (underlying at baseline or developed during tachycardia), could resemble ventricular tachycardia. The clinical setting, characteristics of the arrhythmia, and AV relationship are useful in establishing the diagnosis, although not infrequently an electrophysiology study with intracardiac recordings is needed.
Differential diagnosis
In the differential diagnosis of supraventricular tachycardias, the administration of an intravenous bolus of adenosine (6, 12, or 18 mg administered rapidly and followed by a large bolus of saline flush) may be helpful. If intravenous adenosine does not terminate the tachycardia and results in AV block with continuation of the tachycardia in the atrium, the diagnosis of atrial tachycardia is confirmed.
If the tachycardia terminates with intravenous adenosine, the mechanism of the tachycardia may be suspected as reentrant and possibly with the participation of the AV node but no definitive conclusion can be drawn since some focal atrial tachycardias, as well as macroreentrant atrial tachycardias, could terminate with intravenous adenosine.
Other diagnosis
Other conditions:
- Atrial flutter: the characteristic electrocardiographic pattern and atrial rate around 300 bpm establishes the differential diagnosis. Nevertheless, in practice, there is an overlap between macroreentrant atrial tachycardias and atrial flutter.
By mechanism, typical atrial flutter (a circuit of reentry in the right atrium using the cavo-tricuspid isthmus as the critical isthmus) is a form of macro-reentrant atrial tachycardia and many macro-reentrant atrial tachycardias could be considered forms of atypical atrial flutter, and manifest clinically and in the electrocardiogram as atrial flutter. A definitive diagnosis can only be made in the electrophysiology laboratory with the characterization of the tachycardia circuit.
- Atrial fibrillation: the characteristic electrocardiographic pattern of fibrillatory waves in the electrocardiogram and irregularity of the arrhythmia are diagnostic of atrial fibrillation.
- Inappropriate sinus tachycardia: often difficult to differentiate from an atrial tachycardia. Symptoms, clinical presentation, and patient population are very similar. The electrocardiogram during tachycardia shows P waves identical to the P waves during slower rates. Overlap of the P wave with the preceding T wave may make the comparison of the P waves difficult. An ambulatory heart rhythm monitor to document the progressive acceleration and deceleration of inappropriate sinus tachycardia without a sudden onset or change in P wave morphology is often required.
- Postural orthostatic tachycardia syndrome (POTS): P waves are identical to the P waves in sinus rhythm. The characteristic development of tachycardia and symptoms with upright posture and resolution of the symptoms help differentiate this syndrome from the other forms of supraventricular tachycardias.
- Physiologic sinus tachycardia: the P waves during tachycardia are identical to the P waves at slower rates. An underlying cause for the tachycardia eventually becomes apparent including anemia, volume depletion, hypoxia, pain, anxiety, or a drug effect (i.e., dopamine, albuterol).
- Frequent ectopy: a common reason for consultation; frequent atrial and/or ventricular ectopy could resemble the symptoms of atrial tachycardia and ambulatory rhythm monitoring devices can establish the diagnosis.
D. Physical Examination Findings.
The landmark physical finding in atrial tachycardias is the tachycardia itself, with a heart rate above 100 bpm and rarely dropping below but faster than the underlying sinus rhythm. Frequently, patients have no other physical findings related to the arrhythmia and while not in tachycardia their physical exam may be completely normal. Depending on the physiologic effects and rate of the tachycardia, like with any other supraventricular tachycardia, the patient may present the following symptoms during the arrhythmia:
- Change in blood pressure
- Diaphoresis
- Pallor
- Flushing
- Cold extremities
Underlying conditions that could predispose the development of atrial tachycardias may have more specific physical findings. Heart murmur in the case of valvular heart disease, hypertrophic cardiomyopathy, and congenital heart disease; sustained apical impulse in the case of left ventricular hypertrophy, barrel chest, tachypnea, or adventitious lung sounds in patients with underlying pulmonary disease.
E. What diagnostic tests should be performed?
The physical examination does not provide specific findings confirmatory of the diagnosis of atrial tachycardia. A suspicion is risen by the finding of tachycardia (heart rate >100 bpm) with no plausible physiologic explanation. Vagal maneuvers like Valsalva, carotid sinus massage (limited to patients without suspected carotid occlusive disease), and cold drinks, among others, may terminate the tachycardia and suggest the diagnosis of a supraventricular tachycardia but have no value on the specific diagnosis of an atrial tachycardia.
Sinus tachycardia slows with vagal maneuvers with progressive deceleration/acceleration of the heart rate. Other findings in the physical exam could explain physiologic sinus tachycardia, including hypotension, hypoxia, signs of inflammation/infection, pallor, abdominal distention, or tenderness, etc.
1. What laboratory studies (if any) should be ordered to help establish the diagnosis? How should the results be interpreted?
Laboratory studies may be helpful in the diagnosis of underlying or associated conditions as well as in the differential diagnosis of sinus tachycardia but no laboratory test is useful in the specific diagnosis of an atrial tachycardia. Laboratory blood tests should be based on the physician's clinical judgment after evaluation of the patient's comorbidities, complaints, and physical findings.
As an example, a complete blood count aids in the diagnosis of anemia or potential underlying infections; thyroid function tests help to address the clinical suspicion of thyroid disease; a blood gas analysis determines the presence and severity of hypoxia in predisposing pulmonary conditions; serum level of brain natriuretic peptide complements the evaluation of associated heart failure; and renal function tests and creatinine clearance have implications on the choice of antiarrhythmic drug.
The diagnosis of supraventricular tachycardias and specifically atrial tachycardias is established with the documentation and characterization of the heart rhythm as discussed in section II E 2 below.
2. What imaging studies (if any) should be ordered to help establish the diagnosis? How should the results be interpreted?
Documentation of the tachycardia and evaluation of the underlying cardiac condition are fundamental in the diagnosis and management of this arrhythmia:
ECG: a 12-lead electrocardiogram recorded in sinus rhythm and during tachycardia. P waves during tachycardia exhibiting an axis different than expected for sinus tachycardia and a normal or short P–R interval is compatible with the diagnosis of atrial tachycardia. A similar electrocardiogram can be seen with the so-called atypical (fast-slow or slow-slow) variants of AV nodal reentrant tachycardia and in persistent junctional reciprocating tachycardia (atrioventricular reentrant tachycardia using a slow conducting retrograde accessory pathway).
A long P–R interval during tachycardia does not exclude an atrial tachycardia since atrial tachycardias conducted either over the slow pathway of the AV node, a diseased AV node, or an AV node slowed by drugs or increased vagal tone could result in a prolonged P–R interval during the arrhythmia. Paroxysmal atrial tachycardias are frequently elusive and difficult to document in a 12-lead electrocardiogram (Figure 1 , Figure 3 ).
Heart rhythm monitors: when a 12-lead electrocardiogram has not been recorded during tachycardia, the correlation of the patient's symptoms with an arrhythmia must be made either with an in-hospital or ambulatory heart rhythm monitor. Ambulatory heart rhythm monitors include the traditional Holter monitor (usually limited to 24 to 48 hours of heart rhythm recording), portable continuous tele-monitors with ongoing heart rhythm transmission and recording, snapshot event recorders and event loop recorders, which could be portable or implantable.
Frequency of symptoms, availability and patient/physician preference determines the choice of monitoring modality. These monitors could be single or multichannel and the recording of a sudden onset and offset of tachycardia with P wave morphology different than the one in sinus rhythm is suggestive of the diagnosis of supraventricular tachycardia.
Electrophysiology study: the definitive diagnosis confirmation of an atrial tachycardia is best done in the electrophysiology laboratory. Intracardiac recordings and response to pacing maneuvers during tachycardia, as well as characteristics of tachycardia induction and activation maps, provide a definitive diagnosis of the mechanism of the tachycardia as well as an opportunity for definitive treatment through ablation.
Advanced nonfluoroscopic three-dimensional mapping systems with computerized representation of the cardiac chambers where activation and signal characteristics can be displayed are available today to facilitate the diagnosis and therapy in the electrophysiology laboratory (Figure 2 , Figure 4 ).
Echocardiogram: indicated for the evaluation of potential or known underlying structural heart disease .
III. Management.
The treatment of atrial tachycardias is focused on rhythm control and prevention of arrhythmia recurrence. Persistent tachycardias with hemodynamic compromise must be terminated emergently with electrical cardioversion.
In the case of persistent focal atrial tachycardias and frequently in the management of macro-reentrant atrial tachycardias that resemble atrial flutter, the initial treatment may be aimed at rate control but more definitive treatment and resolution of the clinical symptoms usually requires restoration of normal sinus rhythm.
The treatment could be pharmacologic or catheter based ablation. The patient should participate in the decision of which strategy to use as long as he or she can be a candidate for both. Patients with multiple comorbidities and contraindications for antiarrhythmic drugs should be treated with an invasive strategy, and patients with vascular access problems, contraindication to sedatives, and inability to safely withstand an ablation procedure should be treated with a conservative approach until they can be candidates for an invasive procedure.
Pharmacologic therapy
Pharmacologic therapy includes beta-blockers, calcium channel blockers, and antiarrhythmic drugs. Among antiarrhythmic drugs, class IC seem to be particularly effective with focal atrial tachycardias, but its use is limited to patients without myocardial scar and by extension, patients without coronary artery disease or significant structural heart disease.
Sotalol, amiodarone, and dofetilide can be used as well. Dofetilide have been reported to be more effective in the management of atrial flutter and hence suspected to be more effective for macro-reentrant atrial tachycardias but limited by the QTc and creatinine clearance.
Catheter-based ablation
Treatment through catheter based ablation is the preferred approach given its high success rate and potential freedom of arrhythmias without the need for continued drug therapy. The catheter ablation procedure is a percutaneous invasive intervention that requires vascular access through the femoral and sometimes subclavian veins. Catheters with electrodes are advanced into the heart under fluoroscopy and used for recording intracardiac signals and stimulating the heart.
With the use of pacing maneuvers and pharmacologic interventions, the tachycardia can be induced (if not present at the time of the procedure) and characterized establishing a definitive diagnosis of atrial tachycardia and differentiating between focal and macro-reentrant. Radiofrequency energy or cryoablation are delivered through a catheter tip to eliminate the tissue where a focal atrial tachycardia originates or tissue that is critical for the reentry circuit (Figure 4 , Figure 5 ).
Figure 5.
Surface and intracardiac electrogram recordings during ablation and termination of the macro-reentrant atrial tachycardia shown in Figures 3 and 4. From top to bottom are shown leads I, II, and V1, followed by recordings from the proximal (MAP P)and distal (MAP D)electrodes of the ablation catheter, coronary sinus catheter (CS 4,5, and D) and right ventricular catheter (RV). The asterisks mark the double potentials corresponding to the area of slow conduction functioning as isthmus of the reentrant tachycardia. Once radiofrequency ablation eliminates conduction in the isthmus, only one component of the double potential is recorded at the time of tachycardia termination. Conduction block is created in the area of slow conduction, rendering the tachycardia not inducible.
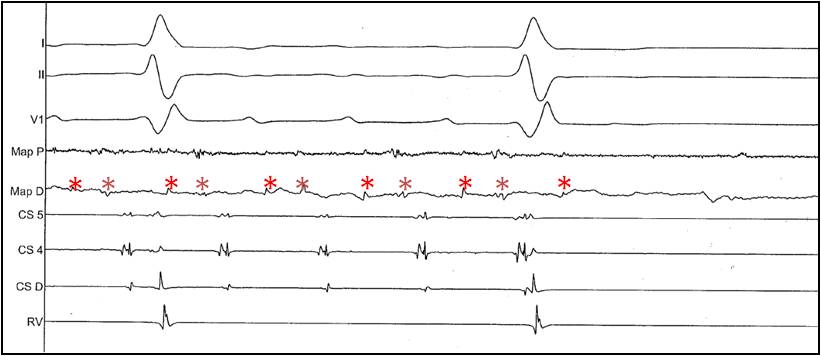
The procedure may require access to the left atrium performing a transseptal puncture or retrograde through the aorta. The contemporary approach to catheter-based ablation using nonfluoroscopic mapping systems, intracardiac echocardiography, and irrigated tip catheters allows for improved safety during the procedure. Success rate is close to 90% and recurrence rates are low.
Macro-reentrant atrial tachycardias associated to extensive atrial fibrosis in patients with valvular heart disease, multiple extensive atrial ablations, or surgeries may be particularly difficult to control both with drugs or ablation and eventually may require an aggressive rate-control strategy with AV junction ablation and the implantation of a permanent pacemaker.
A. Immediate management.
During tachycardia in the acute setting hemodynamic support and restoration of adequate perfusion should remain the priority as mandated by the ACLS protocols. When there is evidence of hemodynamic compromise restoration of normal sinus rhythm and perfusion should be achieved immediately through CPR, electrical cardioversion, and vasoactive drugs.
Intravenous hydration is recommended during tachycardia in the absence of signs and symptoms of congestive heart failure. If the blood pressure is not critically compromised, a bolus of intravenous adenosine can help in establishing the diagnosis or terminating the tachycardia.
Vagal maneuvers including carotid sinus massage are also helpful either in terminating a supraventricular tachycardia or causing transient AV nodal block to allow the diagnosis of an atrial tachycardia when the atrial tachycardia continues despite conduction block to the ventricle.
Antiarrhythmic drugs in the acute setting can also be used for pharmacologic conversion of an incessant tachycardia or to achieve rate control and symptomatic relief. Beta-blockers are the first-line of therapy, particularly if there blood pressure is stable. Other antiarrhythmic drugs include class Ic antiarrhythmics given orally (flecainide 200 to 300 mg, propafenone 600 mg), intravenous amiodarone and ibutilide. Intravenous calcium channel blockers or digoxin can be attempted for rate control, especially in faster tachycardias (macro-reentrant atrial tachycardias that resemble atrial flutter) in which AV nodal blocking agents could be expected to slow ventricular response.
Procainamide, disopyramide, and quinidine are rarely used. Class Ic antiarrhythmic drugs (flecainide, propafenone, procainamide, and disopyramide) should be only given to patients with macro-reentrant atrial tachycardias and atrial flutter when administered together with AV nodal blocking agents to prevent slowing of the tachycardia cycle length and 1:1 AV conduction that could lead to hemodynamic instability.
B. Physical Examination Tips to Guide Management.
Blood pressure and pulse rate should be monitored during the treatment of the arrhythmia in the acute setting, although a heart rhythm monitor or electrocardiogram is of most value. When the tachycardia is accompanied by signs of heart failure, dizziness, or syncope, monitoring respiratory rate, pulse oximetry, body weight, lung sounds, jugular venous distention, peripheral edema, urinary output, and neurologic and mental status is warranted to prevent worsening of those conditions that may be a consequence of coexisting with the tachycardia.
C. Laboratory Tests to Monitor Response To, and Adjustments in, Management
Continuous heart rhythm monitoring and 12-lead electrocardiograms to document and analyze rhythm changes is of most value to monitor the response to therapy.
Laboratory tests are usually not needed unless used to guide response to concomitant therapy for other associated conditions or complications of the arrhythmia:
- Chest radiograph, BNP, electrolytes, and liver function for heart failure.
- Liver function and renal function in case of hypotension.
- Thyroid function in the setting of thyroid disease.
- Renal function for adjustment or dosing of drugs, such as sotalol and dofetilide.
D. Long-term Management.
Catheter ablation is the therapeutic strategy most likely to provide long-term elimination of the arrhythmia. Antiarrhythmic drugs can be used for maintenance of sinus rhythm. Patients may respond to beta-blockers and calcium channel blockers but commonly require the addition of a more potent antiarrhythmic drug, such as sodium (flecainide, propafenone) or potassium (sotalol, amiodarone, dronedarone, dofetilide) channel blockers. Recurrences are possible with both modalities of treatment.
E. Common Pitfalls and Side-Effects of Management
Lack of recognition of the arrhythmia. Incessant atrial tachycardias may be misinterpreted as sinus tachycardia. When incessant atrial tachycardias are not recognized could progress to the development of tachycardia-induced cardiomyopathy and heart failure. Paroxysmal atrial tachycardias may require the use of prolonged ambulatory heart rhythm monitors since recurrence may be sporadic and missed in 24 to 48 hours of ambulatory recordings.
Prevention of recurrence. After termination of the tachycardia in the acute setting, the patient must be educated regarding the recurrent nature of the arrhythmia, and therapy to prevent recurrence should be considered. Patients discharged without a specific therapy or plan for management of recurrences are likely to be readmitted or present to the emergency department or outpatient clinic with recurrence of tachycardia and its symptoms.
Associated conditions. The evaluation and management of atrial tachycardias should include the prompt diagnosis and treatment of associated conditions that could predispose, aggravate, or coexist with the tachycardia. Screening for thyroid, pulmonary, and ischemic heart disease should be considered. Substance abuse, prescription, and over-the-counter drugs (stimulants and decongestants) could be predisposing or aggravating factors. Avoidance of identified triggers if present, such as caffeinated drinks or exercise, should be recommended until an effective therapy is instituted.
Side effects of drug therapy. The side effects are characteristic of each group or agent. The most common side effects include:
- Beta-blockers: bradycardia, fatigue, erectile dysfunction, depression, hypotension, and bronchospasm
- Calcium channel blockers: lower extremity edema, hypotension, and constipation
- Flecainide: nightmares, headaches, paresthesia, and proarrhythmia
- Propafenone: bradycardia, and proarrhythmia.
- Sotalol: bradycardia, Q–T prolongation, and proarrhythmia
- Dofetilide: Q–T prolongation and proarrhythmia
- Amiodarone: bradycardia and nausea, short and long term there is risk of neuropathy, thyroid, lung, and liver toxicity
- Dronedarone: bradycardia, nausea, and diarrhea
Risks of catheter based ablation. The electrophysiology study and catheter-based ablation procedure is an invasive percutaneous procedure that involves vascular access and frequently access to the left atrium. In order to make the diagnosis and deliver the treatment, the tachycardia needs to be present or be inducible. If the arrhythmia cannot be induced during the procedure, neither the diagnostic confirmation nor an ablation can be performed.
Ablation may be required close to vital structures such as coronary arteries, atrioventricular node, sinus node, phrenic nerve, etc. Complications of the procedure include radiation exposure, bleeding, vascular damage, hematoma, arteriovenous fistula, venous thrombosis, pneumothorax, stroke, heart perforation, cardiac tamponade, atrioventricular nodal block, phrenic nerve paralysis, myocardial infarction, and death.
Acutely, a rapid bolus of IV adenosine (6, 12, or 18 mg) can be used for both the differential diagnosis or eventually termination of atrial tachycardias. Only a subset of atrial tachycardias are adenosine sensitive. Intravenous boluses or continuous infusions of beta-blockers, calcium channel blockers, procainamide or amiodarone may help terminate and/or prevent recurrence of the tachycardia. One milligram of IV Ibutilide administered over 10 minutes in a monitor setting with capability of adult cardiac life support may also be used for the acute termination and suppression of the arrhythmia.
The drugs used in the long-term management of atrial tachycardias include beta-blockers, calcium channel blockers, sodium (class IC), and potassium (class III) channel blockers.
Beta-blockers
Most commonly used oral beta-blockers:
- Atenolol 12.5-200 mg daily
- Metoprolol 12.5-100 mg b.i.d.
- Metoprolol extended release 12.5-200 mg daily
- Bisoprolol 5-20 mg daily
- Carvedilol 3.125-25 mg b.i.d
- Carvedilol controlled release 10-80 mg daily
Calcium channel blockers
Most commonly used oral calcium channel blockers:
- Diltiazem 30-120 mg q.i.d.
- Diltiazem sustained/extended release 120-420 mg daily
- Verapamil 40-120 mg t.i.d.
- Verapamil sustained/extended release 100-360 mg daily
Class IC antiarrhythmic drugs (sodium channel blockers)
Class IC antiarrhythmic drugs are contraindicated in patients with myocardial scar, left ventricular hypertrophy, structural heart disease ,and coronary artery disease.
- Flecainide 50-150 mg b.i.d.
- Propafenone 150-300 mg t.i.d.
- Propafenone sustained release 225-425 mg b.i.d.
Class III antiarrhythmic drugs (potassium channel blockers)
Sotalol 40-160 mg b.i.d.—monitoring of the QTc interval during loading for five half-lives (3 days) is recommended
Dofetilide 125-500 mcg b.i.d.—restricted to certified prescribers, requires renal dose adjustment and monitoring of the QTc interval during loading for five half-lives (3 days)
Amiodarone 200-800 mg daily—not recommended for long-term therapy due to potential side effects
Dronedarone 400 mg b.i.d.—may be used off label, but no published literature is available regarding the use of dronedarone in the treatment of supraventricular tachycardias
IV. Management with Co-Morbidities
Thyroid disease. Patients with known thyroid disease on supplementation therapy or uncontrolled hyperthyroidisms may have a higher incidence of atrial tachycardias. Control of hyperthyroidism, including surgical removal or radioactive ablation of the thyroid gland may be necessary. In patients on supplementation therapy, occasionally a reduction on the supplementation dose could help present recurrences. Catheter ablation, if not contraindicated, may be an alternative to allow less aggressive treatment for the thyroid disease.
Coronary artery disease. Patients with coronary artery disease require aggressive treatment of supraventricular tachycardias to prevent angina, heart failure, or myocardial infarction from demand ischemia during tachycardia. Beta-blockers are indicated for coronary artery disease, especially after myocardial infarction. Class IC antiarrhythmic drugs should be avoided in coronary artery disease and are contraindicated in patients with prior to myocardial infarction.
Heart failure. Both systolic and diastolic heart failure could become decompensated during tachycardia. Beta-blockers are indicated in the treatment of heart failure. As previously discussed ,systolic heart failure could result from incessant atrial tachycardias. In the case of tachycardia-induced cardiomyopathy, control of the tachycardia frequently results in normalization of left ventricular function. Class IC antiarrhythmic drugs and dronedarone are in general contraindicated in patients with heart failure.
Congenital heart disease. Atrial tachycardias are rather frequent among patients with history of congenital heart disease. Usually, tachycardia is not well tolerated due to the development of heart failure symptoms or hemodynamic compromise. Atrial tachycardias are commonly macro-reentrant involving scars from previous corrective surgery. Both antiarrhythmic drugs and catheter ablation may be effective in suppressing the arrhythmia. Multiple atrial tachycardias are common in this patient population.
Hypertrophic cardiomyopathy. Atrial tachycardias are poorly tolerated in patients with hypertrophic cardiomyopathy due to the severe degree of diastolic heart failure. Tachycardia may result in heart failure decompensation with pulmonary edema. Multiple atrial tachycardias are also frequent due to severe atrial dilatation, which is frequent in these patients. Class IC antiarrhythmic drugs and sotalol are contraindicated. Dronedarone should be avoided since it is contraindicated in patients with symptomatic heart failure and that is how these patients usually present with atrial tachycardias.
Chronic obstructive pulmonary disease. Atrial tachycardias are common and frequently poorly tolerated in patients with chronic lung disease. Beta-blockers may worsen a reactive airway disease component when present. Amiodarone should be avoided since pulmonary toxicity may be fatal in patients with baseline poor lung function.
Renal insufficiency. Sotalol and dofetilide should be avoided in renal insufficiency. If no safe alternative is available or possible, the patient needs to be carefully monitored and the dose adjusted according to the creatinine clearance. Dofetilide is contraindicated in patients with creatinine clearance <20 ml/min.
Deep venous thrombosis. Thrombosis of both femoral veins or the inferior vena cava prevents vascular access for an electrophysiology study and catheter ablation. Nevertheless, occlusion of the inferior vena cava or bilateral femoral veins is extremely rare. The presence of an inferior vena cava (IVC) filter does not prevent the advance of catheters, which should be avoided only after a recent IVC filter implant to prevent its dislodgement. Pharmacologic suppression should be the treatment of choice for patients with recently implanted IVC filters or acute/subacute femoral vein thrombosis.