Leadless pacing available for selected patients
Over time, the patient population receiving pacing therapy has become older and more complex. The "weak link" in device therapy has been the leads. While transvenous leads typically have lower thresholds and better longevity than epicardial leads, they are associated with increased morbidity and mortality.
Complications at implant include bleeding, vascular damage, cardiac perforation, pneumothorax and dislodgment. Potential long-term concerns include lead fracture, malfunction, venous obstruction, tricuspid valve regurgitation and the risks associated with lead extraction. Transvenous leads are contraindicated in the presence of right-to-left shunt and in some patients with congenital heart disease.
Nanostim leadless pacing device
Micra leadless pacing device
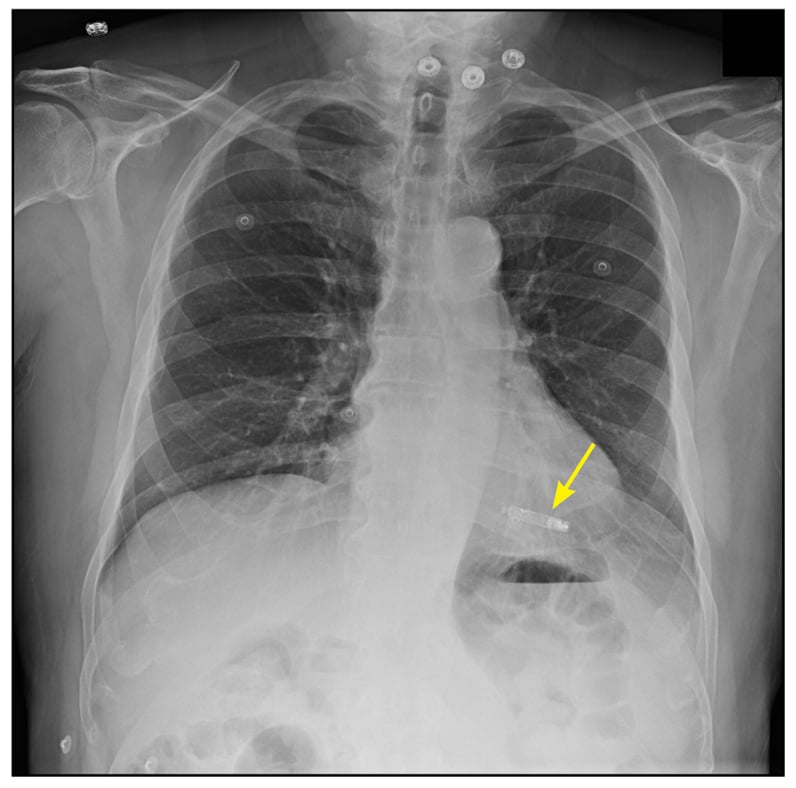
Implanted leadless pacemaker
The concept of leadless pacing was first proposed in 1970 by J. William Spickler, Ph.D., and colleagues, but it is only recently that leadless devices have become available. Currently available are the Nanostim leadless pacemaker (St. Jude Medical, St. Paul, Minnesota) and the Micra transcatheter pacing system (Medtronic, Minneapolis); both are dime-sized capsules that are implanted directly into the right ventricular apex.The Nanostim uses active fixation, while the Micra has a tined fixation mechanism to secure the device to the right ventricular endocardial surface. Both devices are capable of VVIR pacing and have estimated battery longevity between 7 and 10 years. When the battery is depleted, a new device can be implanted and the existing device left in place.
These devices are contraindicated in individuals who require dual-chamber pacing or who have demonstrable pacemaker syndrome. Anticoagulation is not required after implant placement. Current devices are not MRI compatible.
Leadless pacemakers are contraindicated in patients with implantable cardioverter-defibrillators, as high-voltage shocks could damage the pacemaker, and the effect of the pacemaker on shock effectiveness is unknown.
Leadless devices should be avoided in individuals with elevated right ventricular pressures because of higher theoretical risk of embolization. The presence of mechanical tricuspid valves or inferior vena cava filters also precludes the use of leadless pacemakers. Successful device retrieval has been accomplished in animal studies.




No comments:
Post a Comment